| Reticular formation | |
|---|---|

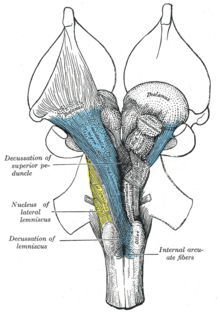
Axial section of the pons, at its upper part. (Formatio reticularis labeled at left.)
| |

Section of the medulla oblongata at about the middle of the olive. (Formatio reticularis grisea and formatio reticularis alba labeled at left.)
| |
| Details | |
| Identifiers | |
| Latin | formatio reticularis |
| MeSH | D012154 |
| NeuroNames | 1223 |
| NeuroLex ID | nlx_143558 |
| TA | A14.1.00.021 A14.1.05.403 A14.1.06.327 |
| FMA | 77719 |
The reticular formation is a set of interconnected nuclei that are located throughout the brainstem. The reticular formation is not anatomically well defined because it includes neurons located in diverse parts of the brain. The neurons of the reticular formation make up a complex set of networks in the core of the brainstem that stretch from the upper part of the midbrain to the lower part of the medulla oblongata. The reticular formation includes ascending pathways to the cortex in the ascending reticular activating system (ARAS) and descending pathways to the spinal cord via the reticulospinal tracts of the descending reticular formation.
Neurons of the reticular formation, particularly those of the ascending reticular activating system, play a crucial role in maintaining behavioral arousal and consciousness. The functions of the reticular formation are modulatory and premotor. The modulatory functions are primarily found in the rostral sector of the reticular formation and the premotor functions are localized in the neurons in more caudal regions.
The reticular formation is divided into three columns: raphe nuclei (median), gigantocellular reticular nuclei (medial zone), and parvocellular reticular nuclei (lateral zone). The raphe nuclei are the place of synthesis of the neurotransmitter serotonin, which plays an important role in mood regulation. The gigantocellular nuclei are involved in motor coordination. The parvocellular nuclei regulate exhalation.
The reticular formation is essential for governing some of the basic functions of higher organisms and is one of the phylogenetically oldest portions of the brain.
General structure
A cross section of the lower part of the pons showing the pontine reticular formation labeled as #9
The human reticular formation is composed of almost 100 brain nuclei and contains many projections into the forebrain, brainstem, and cerebellum, among other regions. It includes the reticular nuclei, reticulothalamic projection fibers, diffuse thalamo-cortical projections, ascending cholinergic projections, descending non-cholinergic projections, and descending reticulospinal projections. The reticular formation also contains two major neural subsystems,
the ascending reticular activating system and descending reticulospinal
tracts, which mediate distinct cognitive and physiological processes. It has been functionally cleaved both sagittally and coronally.
Traditionally the reticular nuclei are divided into three columns:
- In the median column – the raphe nuclei
- In the medial column – gigantocellular nuclei (because of larger size of the cells)
- In the lateral column – parvocellular nuclei (because of smaller size of the cells)
The original functional differentiation was a division of caudal and rostral. This was based upon the observation that the lesioning of the rostral reticular formation induces a hypersomnia in the cat brain. In contrast, lesioning of the more caudal portion of the reticular formation produces insomnia in cats. This study has led to the idea that the caudal portion inhibits the rostral portion of the reticular formation.
Sagittal division reveals more morphological distinctions. The raphe nuclei
form a ridge in the middle of the reticular formation, and, directly to
its periphery, there is a division called the medial reticular
formation. The medial RF is large and has long ascending and descending
fibers, and is surrounded by the lateral reticular formation. The
lateral RF is close to the motor nuclei of the cranial nerves, and
mostly mediates their function.
Medial and lateral reticular formation
The
medial reticular formation and lateral reticular formation are two
columns of neuronal nuclei with ill-defined boundaries that send
projections through the medulla and into the mesencephalon (midbrain). The nuclei can be differentiated by function, cell type, and projections of efferent or afferent nerves. Moving caudally from the rostral midbrain, at the site of the rostral pons and the midbrain, the medial RF becomes less prominent, and the lateral RF becomes more prominent.
Existing on the sides of the medial reticular formation is its lateral
cousin, which is particularly pronounced in the rostral medulla and
caudal pons. Out from this area spring the cranial nerves, including
the very important vagus nerve. The Lateral RF is known for its ganglions and areas of interneurons around the cranial nerves, which serve to mediate their characteristic reflexes and functions.
General functions
The reticular formation consists of more than 100 small neural networks, with varied functions including the following:
- Somatic motor control – Some motor neurons send their axons to the reticular formation nuclei, giving rise to the reticulospinal tracts of the spinal cord. These tracts function in maintaining tone, balance, and posture—especially during body movements. The reticular formation also relays eye and ear signals to the cerebellum so that the cerebellum can integrate visual, auditory, and vestibular stimuli in motor coordination. Other motor nuclei include gaze centers, which enable the eyes to track and fixate objects, and central pattern generators, which produce rhythmic signals of breathing with swallowing, and with defecation and urination.
- Cardiovascular control – The reticular formation includes the cardiac and vasomotor centers of the medulla oblongata.
- Pain modulation – The reticular formation is one means by which pain signals from the lower body reach the cerebral cortex. It is also the origin of the descending analgesic pathways. The nerve fibers in these pathways act in the spinal cord to block the transmission of some pain signals to the brain.
- Sleep and consciousness – The reticular formation has projections to the thalamus and cerebral cortex that allow it to exert some control over which sensory signals reach the cerebrum and come to our conscious attention. It plays a central role in states of consciousness like alertness and sleep. Injury to the reticular formation can result in irreversible coma.
- Habituation – This is a process in which the brain learns to ignore repetitive, meaningless stimuli while remaining sensitive to others. A good example of this is a person who can sleep through loud traffic in a large city, but is awakened promptly due to the sound of an alarm or crying baby. Reticular formation nuclei that modulate activity of the cerebral cortex are part of the ascending reticular activating system.
Major subsystems
Ascending reticular activating system
Ascending reticular activating system. Reticular formation labeled near center.
The ascending reticular activating system (ARAS), also known as the extrathalamic control modulatory system or simply the reticular activating system (RAS), is a set of connected nuclei in the brains of vertebrates that is responsible for regulating wakefulness and sleep-wake transitions. The ARAS is a part of the reticular formation and is mostly composed of various nuclei in the thalamus and a number of dopaminergic, noradrenergic, serotonergic, histaminergic, cholinergic, and glutamatergic brain nuclei.
Structure of the ARAS
The ARAS is composed of several neuronal circuits connecting the dorsal part of the posterior midbrain and anterior pons to the cerebral cortex via distinct pathways that project through the thalamus and hypothalamus.
The ARAS is a collection of different nuclei – more than 20 on each
side in the upper brainstem, the pons, medulla, and posterior
hypothalamus. The neurotransmitters that these neurons release include dopamine, norepinephrine, serotonin, histamine, acetylcholine, and glutamate. They exert cortical influence through direct axonal projections and indirect projections through thalamic relays.
The thalamic pathway consists primarily of cholinergic neurons in the pontine tegmentum, whereas the hypothalamic pathway is composed primarily of neurons that release monoamine neurotransmitters, namely dopamine, norepinephrine, serotonin, and histamine.
The glutamate-releasing neurons in the ARAS were identified much more
recently relative to the monoaminergic and cholinergic nuclei;
the glutamatergic component of the ARAS includes one glutamatergic
nucleus in the hypothalamus and various glutamatergic brainstem nuclei. The orexin neurons of the lateral hypothalamus innervate every component of the ascending reticular activating system and coordinate activity within the entire system.
The key components of the ARAS are listed in the table below.
| Nucleus type | Corresponding nuclei that mediate arousal |
|---|---|
| Dopaminergic nuclei | |
| Noradrenergic nuclei |
|
| Serotonergic nuclei | |
| Histaminergic nuclei | |
| Cholinergic nuclei | |
| Glutamatergic nuclei |
|
| Thalamic nuclei |
|
The ARAS consists of evolutionarily ancient areas of the brain, which
are crucial to survival and protected during adverse periods. As a
result, the ARAS still functions during inhibitory periods of hypnosis.
The ascending reticular activating system which sends neuromodulatory projections to the cortex - mainly connects to the prefrontal cortex. There is seen to be low connectivity to the motor areas of the cortex.
Functions of the ARAS
Consciousness
The ascending reticular activating system is an important enabling factor for the state of consciousness. The ascending system is seen to contribute to wakefulness as characterised by cortical and behavioural arousal.
Regulating sleep-wake transitions
The main function of the ARAS is to modify and potentiate thalamic and cortical function such that electroencephalogram (EEG) desynchronization ensues.
There are distinct differences in the brain's electrical activity
during periods of wakefulness and sleep: Low voltage fast burst brain waves (EEG desynchronization) are associated with wakefulness and REM sleep
(which are electrophysiologically similar); high voltage slow waves are
found during non-REM sleep. Generally speaking, when thalamic relay
neurons are in burst mode the EEG is synchronized and when they are in tonic mode it is desynchronized. Stimulation of the ARAS produces EEG desynchronization by suppressing slow cortical waves (0.3–1 Hz), delta waves (1–4 Hz), and spindle wave oscillations (11–14 Hz) and by promoting gamma band (20 – 40 Hz) oscillations.
The physiological change from a state of deep sleep to wakefulness is reversible and mediated by the ARAS. Inhibitory influence from the brain is active at sleep onset, likely coming from the preoptic area
(POA) of the hypothalamus. During sleep, neurons in the ARAS will have a
much lower firing rate; conversely, they will have a higher activity
level during the waking state.
Therefore, low frequency inputs (during sleep) from the ARAS to the POA
neurons result in an excitatory influence and higher activity levels
(awake) will have inhibitory influence. In order that the brain may
sleep, there must be a reduction in ascending afferent activity reaching
the cortex by suppression of the ARAS.
Attention
The ARAS also helps mediate transitions from relaxed wakefulness to periods of high attention.
There is increased regional blood flow (presumably indicating an
increased measure of neuronal activity) in the midbrain reticular
formation (MRF) and thalamic intralaminar nuclei during tasks requiring
increased alertness and attention.
Clinical significance of the ARAS
Mass lesions in brainstem ARAS nuclei can cause severe alterations in level of consciousness (e.g., coma). Bilateral damage to the reticular formation of the midbrain may lead to coma or death.
Direct electrical stimulation of the ARAS produces pain responses in cats and educes verbal reports of pain in humans. Additionally, ascending reticular activation in cats can produce mydriasis,
which can result from prolonged pain. These results suggest some
relationship between ARAS circuits and physiological pain pathways.
Pathologies
Given
the importance of the ARAS for modulating cortical changes, disorders
of the ARAS should result in alterations of sleep-wake cycles and
disturbances in arousal.
Some pathologies of the ARAS may be attributed to age, as there
appears to be a general decline in reactivity of the ARAS with advancing
years.
Changes in electrical coupling have been suggested to account for some
changes in ARAS activity: If coupling were down-regulated, there would
be a corresponding decrease in higher-frequency synchronization (gamma
band). Conversely, up-regulated electrical coupling would increase
synchronization of fast rhythms that could lead to increased arousal and
REM sleep drive. Specifically, disruption of the ARAS has been implicated in the following disorders:
- Narcolepsy: Lesions along the PPT/LDT nuclei are associated with narcolepsy. There is a significant down-regulation of PPN output and a loss of orexin peptides, promoting the excessive daytime sleepiness that is characteristic of this disorder.
- Schizophrenia: Intractable schizophrenic patients have a significant increase (> 60%) in the number of PPN neurons and dysfunction of NO signaling involved in modulating cholinergic output of the ARAS.
- Post-traumatic stress disorder, Parkinson's disease, REM behavior disorder: Patients with these syndromes exhibit a significant (>50%) decrease in the number of locus coeruleus (LC) neurons, resulting is increased disinhibition of the PPN.
- Progressive supranuclear palsy (PSP): Dysfunction of NO signaling has been implicated in the development of PSP.
- Depression, autism, Alzheimer's disease, attention deficit disorder: The exact role of the ARAS in each of these disorders has not yet been identified. However, it is expected that in any neurological or psychiatric disease that manifests disturbances in arousal and sleep-wake cycle regulation, there will be a corresponding dysregulation of some elements of the ARAS.
- Parkinson's disease: REM sleep disturbances are common in Parkinson's. It is mainly a dopaminergic disease, but cholinergic nuclei are depleted as well. Degeneration in the ARAS begins early in the disease process.
Developmental influences
There are several potential factors that may adversely influence the development of the ascending reticular activating system:
- Preterm birth: Regardless of birth weight or weeks of gestation, premature birth induces persistent deleterious effects on pre-attentional (arousal and sleep-wake abnormalities), attentional (reaction time and sensory gating), and cortical mechanisms throughout development.
- Smoking during pregnancy: Prenatal exposure to cigarette smoke is known to produce lasting arousal, attentional and cognitive deficits in humans. This exposure can induce up-regulation of nicotinic receptors on α4b2 subunit on Pedunculopontine nucleus (PPN) cells, resulting in increased tonic activity, resting membrane potential, and hyperpolarization-activated cation current. These major disturbances of the intrinsic membrane properties of PPN neurons result in increased levels of arousal and sensory gating deficits (demonstrated by a diminished amount of habituation to repeated auditory stimuli). It is hypothesized that these physiological changes may intensify attentional dysregulation later in life.
Descending reticulospinal tracts
Spinal cord tracts - reticulospinal tract labeled in red, near-center at left in figure.
The reticulospinal tracts, also known as the descending or anterior reticulospinal tracts, are extrapyramidal motor tracts that descend from the reticular formation
in two tracts to act on the motor neurons supplying the trunk and
proximal limb flexors and extensors. The reticulospinal tracts are
involved mainly in locomotion and postural control, although they do
have other functions as well.
The descending reticulospinal tracts are one of four major cortical
pathways to the spinal cord for musculoskeletal activity. The
reticulospinal tracts works with the other three pathways to give a
coordinated control of movement, including delicate manipulations.
The four pathways can be grouped into two main system pathways – a
medial system and a lateral system. The medial system includes the
reticulospinal pathway and the vestibulospinal pathway, and this system provides control of posture. The corticospinal and the rubrospinal tract pathways belong to the lateral system which provides fine control of movement.
Components of the reticulospinal tracts
The tract is divided into two parts, the medial (or pontine) and lateral (or medullary) reticulospinal tracts (MRST and LRST).
- The MRST is responsible for exciting anti-gravity, extensor muscles. The fibers of this tract arise from the caudal pontine reticular nucleus and the oral pontine reticular nucleus and project to the lamina VII and lamina VIII of the spinal cord (BrainInfo)
- The LRST is responsible for inhibiting excitatory axial extensor muscles of movement. It is also responsible for automatic breathing. The fibers of this tract arise from the medullary reticular formation, mostly from the gigantocellular nucleus, and descend the length of the spinal cord in the anterior part of the lateral column. The tract terminates in lamina VII mostly with some fibers terminating in lamina IX of the spinal cord.
The ascending sensory tract conveying information in the opposite direction is known as the spinoreticular tract.
Functions of the reticulospinal tracts
- Integrates information from the motor systems to coordinate automatic movements of locomotion and posture
- Facilitates and inhibits voluntary movement; influences muscle tone
- Mediates autonomic functions
- Modulates pain impulses
- Influences blood flow to lateral geniculate nucleus of the thalamus.
Clinical significance of the reticulospinal tracts
The reticulospinal tracts are mostly inhibited by the corticospinal tract; if damage occurs at the level of or below the red nucleus (e.g. to the superior colliculus), it is called decerebration, and causes decerebrate rigidity: an unopposed extension of the head and limbs.
The reticulospinal tracts also provide a pathway by which the
hypothalamus can control sympathetic thoracolumbar outflow and
parasympathetic sacral outflow.[citation needed]
History
The term "reticular formation" was coined in the late 19th century by Otto Deiters, coinciding with Ramon y Cajal’s neuron doctrine. Allan Hobson states in his book The Reticular Formation Revisited that the name is an etymological vestige from the fallen era of the aggregate field theory in the neural sciences. The term "reticulum"
means "netlike structure", which is what the reticular formation
resembles at first glance. It has been described as being either too
complex to study or an undifferentiated part of the brain with no
organization at all. Eric Kandel
describes the reticular formation as being organized in a similar
manner to the intermediate gray matter of the spinal cord. This chaotic,
loose, and intricate form of organization is what has turned off many
researchers from looking farther into this particular area of the brain.
The cells lack clear ganglionic boundaries, but do have clear
functional organizations and distinct cell types. The term "reticular
formation" is seldom used anymore except to speak in generalities.
Modern scientists usually refer to the individual nuclei that compose
the reticular formation.
Moruzzi and Magoun
first investigated the neural components regulating the brain's
sleep-wake mechanisms in 1949. Physiologists had proposed that some
structure deep within the brain controlled mental wakefulness and
alertness. It had been thought that wakefulness depended only on the direct reception of afferent (sensory) stimuli at the cerebral cortex.
The direct electrical stimulation of the brain could simulate
electrocortical relays. Magoun used this principle to demonstrate, on
two separate areas of the brainstem of a cat, how to produce wakefulness
from sleep. First the ascending somatic and auditory paths; second, a series of "ascending relays from the reticular formation of the lower brain stem through the midbrain tegmentum, subthalamus and hypothalamus to the internal capsule."
The latter was of particular interest, as this series of relays did not
correspond to any known anatomical pathways for the wakefulness signal
transduction and was coined the ascending reticular activating system (ARAS).
Next, the significance of this newly identified relay system was evaluated by placing lesions in the medial and lateral portions of the front of the midbrain.
Cats with mesancephalic interruptions to the ARAS entered into a deep
sleep and displayed corresponding brain waves. In alternative fashion,
cats with similarly placed interruptions to ascending auditory and
somatic pathways exhibited normal sleeping and wakefulness, and could be
awakened with somatic stimuli. Because these external stimuli would be
blocked by the interruptions, this indicated that the ascending
transmission must travel through the newly discovered ARAS.
Finally, Magoun recorded potentials within the medial portion of
the brain stem and discovered that auditory stimuli directly fired
portions of the reticular activating system. Furthermore, single-shock
stimulation of the sciatic nerve also activated the medial reticular formation, hypothalamus, and thalamus.
Excitation of the ARAS did not depend on further signal propagation
through the cerebellar circuits, as the same results were obtained
following decerebellation and decortication. The researchers proposed
that a column of cells surrounding the midbrain reticular formation
received input from all the ascending tracts of the brain stem and
relayed these afferents to the cortex and therefore regulated
wakefulness.