Example of a GC-MS instrument
Gas chromatography–mass spectrometry (GC-MS) is an analytical method that combines the features of gas-chromatography and mass spectrometry to identify different substances within a test sample. Applications of GC-MS include drug detection, fire investigation, environmental analysis, explosives
investigation, and identification of unknown samples, including that of
material samples obtained from planet Mars during probe missions as
early as the 1970s. GC-MS can also be used in airport security to detect
substances in luggage or on human beings. Additionally, it can identify
trace elements in materials that were previously thought to have disintegrated beyond identification. Like liquid chromatography–mass spectrometry, it allows analysis and detection even of tiny amounts of a substance.
GC-MS has been regarded as a "gold standard" for forensic substance identification because it is used to perform a 100% specific
test, which positively identifies the presence of a particular
substance. A nonspecific test merely indicates that any of several in a
category of substances is present. Although a nonspecific test could
statistically suggest the identity of the substance, this could lead to false positive identification.
History
The first on-line coupling of gas chromatography to a mass spectrometer was reported in 1959.
The development of affordable and miniaturized computers
has helped in the simplification of the use of this instrument, as well
as allowed great improvements in the amount of time it takes to analyze
a sample. In 1964, Electronic Associates, Inc. (EAI), a leading U.S. supplier of analog computers, began development of a computer controlled quadrupole mass spectrometer under the direction of Robert E. Finnigan. By 1966 Finnigan and collaborator Mike Uthe's EAI division had sold over 500 quadrupole residual gas-analyzer instruments.
In 1967, Finnigan left EAI to form the Finnigan Instrument Corporation
along with Roger Sant, T. Z. Chou, Michael Story, and William Fies. In early 1968, they delivered the first prototype quadrupole GC/MS instruments to Stanford and Purdue University. When Finnigan Instrument Corporation was acquired by Thermo Instrument Systems (later Thermo Fisher Scientific) in 1990, it was considered "the world's leading manufacturer of mass spectrometers".
Instrumentation
The insides of the GC-MS, with the column of the gas chromatograph in the oven on the right.
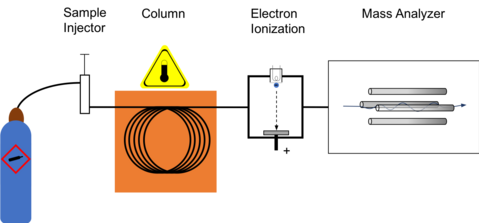
The GC-MS is composed of two major building blocks: the gas chromatograph and the mass spectrometer.
The gas chromatograph utilizes a capillary column which depends on the
column's dimensions (length, diameter, film thickness) as well as the
phase properties (e.g. 5% phenyl polysiloxane). The difference in the
chemical properties between different molecules
in a mixture and their relative affinity for the stationary phase of
the column will promote separation of the molecules as the sample
travels the length of the column. The molecules are retained by the
column and then elute (come off) from the column at different times
(called the retention time), and this allows the mass spectrometer
downstream to capture, ionize, accelerate, deflect, and detect the
ionized molecules separately. The mass spectrometer does this by
breaking each molecule into ionized fragments and detecting these fragments using their mass-to-charge ratio.
GC-MS schematic
These two components, used together, allow a much finer degree of
substance identification than either unit used separately. It is not
possible to make an accurate identification of a particular molecule by
gas chromatography or mass spectrometry alone. The mass spectrometry
process normally requires a very pure sample while gas chromatography
using a traditional detector (e.g. Flame ionization detector) cannot differentiate between multiple molecules that happen to take the same amount of time to travel through the column (i.e.
have the same retention time), which results in two or more molecules
that co-elute. Sometimes two different molecules can also have a similar
pattern of ionized fragments in a mass spectrometer (mass spectrum).
Combining the two processes reduces the possibility of error, as it is
extremely unlikely that two different molecules will behave in the same
way in both a gas chromatograph and a mass spectrometer. Therefore, when
an identifying mass spectrum appears at a characteristic retention time
in a GC-MS analysis, it typically increases certainty that the analyte
of interest is in the sample.
Purge and trap GC-MS
For the analysis of volatile compounds, a purge and trap
(P&T) concentrator system may be used to introduce samples. The
target analytes are extracted by mixing the sample with water and purge
with inert gas (e.g. Nitrogen gas) into an airtight chamber, this is known as purging or sparging. The volatile compounds move into the headspace above the water and are drawn along a pressure gradient
(caused by the introduction of the purge gas) out of the chamber. The
volatile compounds are drawn along a heated line onto a 'trap'. The
trap is a column of adsorbent
material at ambient temperature that holds the compounds by returning
them to the liquid phase. The trap is then heated and the sample
compounds are introduced to the GC-MS column via a volatiles interface,
which is a split inlet system. P&T GC-MS is particularly suited to volatile organic compounds (VOCs) and BTEX compounds (aromatic compounds associated with petroleum).
A faster alternative is the "purge-closed loop" system. In this
system the inert gas is bubbled through the water until the
concentrations of organic compounds in the vapor phase are at
equilibrium with concentrations in the aqueous phase. The gas phase is
then analysed directly.
Types of mass spectrometer detectors
The
most common type of mass spectrometer (MS) associated with a gas
chromatograph (GC) is the quadrupole mass spectrometer, sometimes
referred to by the Hewlett-Packard (now Agilent)
trade name "Mass Selective Detector" (MSD). Another relatively common
detector is the ion trap mass spectrometer. Additionally one may find a
magnetic sector mass spectrometer, however these particular instruments
are expensive and bulky and not typically found in high-throughput
service laboratories. Other detectors may be encountered such as time
of flight (TOF), tandem quadrupoles (MS-MS) (see below), or in the case
of an ion trap MSn where n indicates the number mass spectrometry stages.
GC-tandem MS
When
a second phase of mass fragmentation is added, for example using a
second quadrupole in a quadrupole instrument, it is called tandem MS
(MS/MS). MS/MS can sometimes be used to quantitate low levels of target
compounds in the presence of a high sample matrix background.
The first quadrupole (Q1) is connected with a collision cell (Q2)
and another quadrupole (Q3). Both quadrupoles can be used in scanning
or static mode, depending on the type of MS/MS analysis being performed.
Types of analysis include product ion scan, precursor ion scan, selected reaction monitoring (SRM)
(sometimes referred to as multiple reaction monitoring (MRM)) and
neutral loss scan. For example: When Q1 is in static mode (looking at
one mass only as in SIM), and Q3 is in scanning mode, one obtains a
so-called product ion spectrum (also called "daughter spectrum"). From
this spectrum, one can select a prominent product ion which can be the
product ion for the chosen precursor ion. The pair is called a
"transition" and forms the basis for SRM. SRM is highly specific and
virtually eliminates matrix background.
Ionization
After
the molecules travel the length of the column, pass through the
transfer line and enter into the mass spectrometer they are ionized by
various methods with typically only one method being used at any given
time. Once the sample is fragmented it will then be detected, usually
by an electron multiplier, which essentially turns the ionized mass fragment into an electrical signal that is then detected.
The ionization technique chosen is independent of using full scan or SIM.
Block diagram for gas chromatography using electron ionization for collecting mass spectrum.
Electron ionization
By far the most common and perhaps standard form of ionization is electron ionization
(EI). The molecules enter into the MS (the source is a quadrupole or
the ion trap itself in an ion trap MS) where they are bombarded with
free electrons emitted from a filament, not unlike the filament one
would find in a standard light bulb. The electrons bombard the
molecules, causing the molecule to fragment in a characteristic and
reproducible way. This "hard ionization" technique results in the
creation of more fragments of low mass-to-charge ratio (m/z) and few, if
any, molecules approaching the molecular mass unit. Hard ionization is
considered by mass spectrometrists as the employ of molecular electron
bombardment, whereas "soft ionization" is charge by molecular collision
with an introduced gas. The molecular fragmentation pattern is
dependent upon the electron energy applied to the system, typically 70
eV (electron Volts). The use of 70 eV facilitates comparison of
generated spectra with library spectra using manufacturer-supplied
software or software developed by the National Institute of Standards
(NIST-USA). Spectral library searches employ matching algorithms such
as Probability Based Matching and dot-product
matching that are used with methods of analysis written by many method
standardization agencies. Sources of libraries include NIST, Wiley, the AAFS, and instrument manufacturers.
Cold electron ionization
The "hard ionization" process of electron ionization
can be softened by the cooling of the molecules before their
ionization, resulting in mass spectra that are richer in information.
In this method named cold electron ionization (cold-EI) the molecules
exit the GC column, mixed with added helium make up gas and expand into
vacuum through a specially designed supersonic nozzle, forming a
supersonic molecular beam (SMB). Collisions with the make up gas at the
expanding supersonic jet reduce the internal vibrational (and
rotational) energy of the analyte molecules, hence reducing the degree
of fragmentation caused by the electrons during the ionization process.
Cold-EI mass spectra are characterized by an abundant molecular ion
while the usual fragmentation pattern is retained, thus making cold-EI
mass spectra compatible with library search identification techniques.
The enhanced molecular ions increase the identification probabilities of
both known and unknown compounds, amplify isomer mass spectral effects
and enable the use of isotope abundance analysis for the elucidation of
elemental formulae.
Chemical ionization
In chemical ionization a reagent gas, typically methane or ammonia
is introduced into the mass spectrometer. Depending on the technique
(positive CI or negative CI) chosen, this reagent gas will interact with
the electrons and analyte and cause a 'soft' ionization of the molecule
of interest. A softer ionization fragments the molecule to a lower
degree than the hard ionization of EI. One of the main benefits of
using chemical ionization is that a mass fragment closely corresponding
to the molecular weight of the analyte of interest is produced.
In positive chemical ionization (PCI) the reagent gas interacts
with the target molecule, most often with a proton exchange. This
produces the species in relatively high amounts.
In negative chemical ionization (NCI) the reagent gas decreases
the impact of the free electrons on the target analyte. This decreased
energy typically leaves the fragment in great supply.
Analysis
A
mass spectrometer is typically utilized in one of two ways: full scan or
selective ion monitoring (SIM). The typical GC-MS instrument is
capable of performing both functions either individually or
concomitantly, depending on the setup of the particular instrument.
The primary goal of instrument analysis is to quantify an amount
of substance. This is done by comparing the relative concentrations
among the atomic masses in the generated spectrum. Two kinds of analysis
are possible, comparative and original. Comparative analysis
essentially compares the given spectrum to a spectrum library to see if
its characteristics are present for some sample in the library. This is
best performed by a computer
because there are a myriad of visual distortions that can take place
due to variations in scale. Computers can also simultaneously correlate
more data (such as the retention times identified by GC), to more
accurately relate certain data. Deep learning was shown to lead to
promising results in the identification of VOCs from raw GC-MS data.
Another method of analysis measures the peaks in relation to one
another. In this method, the tallest peak is assigned 100% of the
value, and the other peaks being assigned proportionate values. All
values above 3% are assigned. The total mass of the unknown compound is
normally indicated by the parent peak. The value of this parent peak can
be used to fit with a chemical formula containing the various elements which are believed to be in the compound. The isotope
pattern in the spectrum, which is unique for elements that have many
natural isotopes, can also be used to identify the various elements
present. Once a chemical formula has been matched to the spectrum, the
molecular structure and bonding can be identified, and must be
consistent with the characteristics recorded by GC-MS. Typically, this
identification is done automatically by programs which come with the
instrument, given a list of the elements which could be present in the
sample.
A “full spectrum” analysis considers all the “peaks” within a
spectrum. Conversely, selective ion monitoring (SIM) only monitors
selected ions associated with a specific substance. This is done on the
assumption that at a given retention time, a set of ions
is characteristic of a certain compound. This is a fast and efficient
analysis, especially if the analyst has previous information about a
sample or is only looking for a few specific substances. When the amount
of information collected about the ions in a given gas chromatographic
peak decreases, the sensitivity of the analysis increases. So, SIM
analysis allows for a smaller quantity of a compound to be detected and
measured, but the degree of certainty about the identity of that
compound is reduced.
Full scan MS
When
collecting data in the full scan mode, a target range of mass fragments
is determined and put into the instrument's method. An example of a
typical broad range of mass fragments to monitor would be m/z 50 to m/z
400. The determination of what range to use is largely dictated by
what one anticipates being in the sample while being cognizant of the
solvent and other possible interferences. A MS should not be set to
look for mass fragments too low or else one may detect air (found as m/z 28 due to nitrogen), carbon dioxide (m/z
44) or other possible interference. Additionally if one is to use a
large scan range then sensitivity of the instrument is decreased due to
performing fewer scans per second since each scan will have to detect a
wide range of mass fragments.
Full scan is useful in determining unknown compounds in a sample.
It provides more information than SIM when it comes to confirming or
resolving compounds in a sample. During instrument method development
it may be common to first analyze test solutions in full scan mode to
determine the retention time and the mass fragment fingerprint before
moving to a SIM instrument method.
Selective ion monitoring
In
selective ion monitoring (SIM) certain ion fragments are entered into
the instrument method and only those mass fragments are detected by the
mass spectrometer. The advantages of SIM are that the detection limit
is lower since the instrument is only looking at a small number of
fragments (e.g. three fragments) during each scan. More scans can take
place each second. Since only a few mass fragments of interest are
being monitored, matrix interferences
are typically lower. To additionally confirm the likelihood of a
potentially positive result, it is relatively important to be sure that
the ion ratios of the various mass fragments are comparable to a known
reference standard.
Applications
Environmental monitoring and cleanup
GC-MS
is becoming the tool of choice for tracking organic pollutants in the
environment. The cost of GC-MS equipment has decreased significantly,
and the reliability has increased at the same time, which has
contributed to its increased adoption in environmental studies.
Criminal forensics
GC-MS can analyze the particles from a human body in order to help link a criminal to a crime. The analysis of fire
debris using GC-MS is well established, and there is even an
established American Society for Testing and Materials (ASTM) standard
for fire debris analysis. GCMS/MS is especially useful here as samples
often contain very complex matrices and results, used in court, need to
be highly accurate.
Law enforcement
GC-MS is increasingly used for detection of illegal narcotics, and may eventually supplant drug-sniffing dogs.[1]
A simple and selective GC-MS method for detecting marijuana usage was
recently developed by the Robert Koch-Institute in Germany. It involves
identifying an acid metabolite of tetrahyhydrocannabinol (THC), the
active ingredient in marijuana, in urine samples by employing
derivatization in the sample preparation.
GC-MS is also commonly used in forensic toxicology to find drugs and/or
poisons in biological specimens of suspects, victims, or the deceased.
In drug screening, GC-MS methods frequently utilize liquid-liquid
extraction as a part of sample preparation, in which target compounds
are extracted from blood plasma.
Sports anti-doping analysis
GC-MS
is the main tool used in sports anti-doping laboratories to test
athletes' urine samples for prohibited performance-enhancing drugs, for
example anabolic steroids.
Security
A post–September 11 development, explosive detection systems have become a part of all US airports. These systems run on a host of technologies, many of them based on GC-MS. There are only three manufacturers certified by the FAA to provide these systems, one of which is Thermo Detection (formerly Thermedics), which produces the EGIS,
a GC-MS-based line of explosives detectors. The other two manufacturers
are Barringer Technologies, now owned by Smith 's Detection Systems,
and Ion Track Instruments, part of General Electric Infrastructure
Security Systems.
Chemical warfare agent detection
As
part of the post-September 11 drive towards increased capability in
homeland security and public health preparedness, traditional GC-MS
units with transmission quadrupole mass spectrometers, as well as those
with cylindrical ion trap (CIT-MS) and toroidal ion trap (T-ITMS) mass
spectrometers have been modified for field portability and near
real-time detection of chemical warfare agents (CWA) such as sarin,
soman, and VX.
These complex and large GC-MS systems have been modified and
configured with resistively heated low thermal mass (LTM) gas
chromatographs that reduce analysis time to less than ten percent of the
time required in traditional laboratory systems.
Additionally, the systems are smaller, and more mobile, including
units that are mounted in mobile analytical laboratories (MAL), such as
those used by the United States Marine Corps Chemical and Biological
Incident Response Force MAL and other similar laboratories, and systems
that are hand-carried by two-person teams or individuals, much ado to
the smaller mass detectors. Depending on the system, the analytes can be introduced via liquid injection, desorbed from sorbent tubes through a thermal desorption process, or with solid-phase micro extraction (SPME).
Chemical engineering
GC-MS
is used for the analysis of unknown organic compound mixtures. One
critical use of this technology is the use of GC-MS to determine the
composition of bio-oils processed from raw biomass.
Food, beverage and perfume analysis
Foods and beverages contain numerous aromatic compounds,
some naturally present in the raw materials and some forming during
processing. GC-MS is extensively used for the analysis of these
compounds which include esters, fatty acids, alcohols, aldehydes, terpenes etc. It is also used to detect and measure contaminants from spoilage or adulteration which may be harmful and which is often controlled by governmental agencies, for example pesticides.
Astrochemistry
Several GC-MS have left earth. Two were brought to Mars by the Viking program. Venera 11 and 12 and Pioneer Venus analysed the atmosphere of Venus with GC-MS. The Huygens probe of the Cassini–Huygens mission landed one GC-MS on Saturn's largest moon, Titan. The material in the comet 67P/Churyumov–Gerasimenko will be analysed by the Rosetta mission with a chiral GC-MS in 2014.
Medicine
Dozens of congenital metabolic diseases also known as inborn errors of metabolism (IEM) are now detectable by newborn screening
tests, especially the testing using gas chromatography–mass
spectrometry. GC-MS can determine compounds in urine even in minor
concentration. These compounds are normally not present but appear in
individuals suffering with metabolic disorders. This is increasingly
becoming a common way to diagnose IEM for earlier diagnosis and
institution of treatment eventually leading to a better outcome. It is
now possible to test a newborn for over 100 genetic metabolic disorders
by a urine test at birth based on GC-MS.
In combination with isotopic labeling of metabolic compounds, the GC-MS is used for determining metabolic activity. Most applications are based on the use of 13C as the labeling and the measurement of 13C-12C ratios with an isotope ratio mass spectrometer (IRMS); an MS with a detector designed to measure a few select ions and return values as ratios.