| |

| |
| Names | |
|---|---|
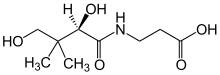
| Preferred IUPAC name
3-[(2R)-2,4-Dihydroxy-3,3-dimethylbutanamido]propanoic acid
| |
| Systematic IUPAC name
3-[(2R)-(2,4-Dihydroxy-3,3-dimethylbutanoyl)amino]propanoic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| 3DMet | B00193 |
| 1727062, 1727064 (R) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.009.061 |
| EC Number | 209-965-4 |
| KEGG | |
| MeSH | Pantothenic+Acid |
PubChem CID
|
|
| RTECS number | RU4729000 |
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| C9H17NO5 | |
| Molar mass | 219.237 g·mol−1 |
| Appearance | Yellow oil Colorless crystals (Ca2+ salt) |
| Odor | Odorless |
| Density | 1.266 g/cm3 1.32 g/cm3 (Ca2+ salt) |
| Melting point | 183.833 °C (362.899 °F; 456.983 K) 196–200 °C (385–392 °F; 469–473 K) decomposes (Ca2+ salt) 138 °C (280 °F; 411 K) decomposes (Ca2+ salt, monohydrate) |
| Very soluble 2.11 g/mL (Ca2+ salt) | |
| Solubility | Very soluble in C6H6, ether Ca2+ salt: Slightly soluble in alcohol, CHCl3 |
| log P | −1.416 |
| Acidity (pKa) | 4.41 |
| Basicity (pKb) | 9.698 |
Chiral rotation ([α]D)
|
+37.5° +24.3° (Ca2+ salt) |
| Hazards | |
| NFPA 704 | |
| Flash point | 287.3 °C (549.1 °F; 560.5 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
> 10 mg/g (Ca2+ salt) |
| Related compounds | |
Related alkanoic acids
|
Arginine Hopantenic acid 4-(γ-Glutamylamino)butanoic acid |
Related compounds
|
Panthenol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pantothenic acid, also called vitamin B5 (a B vitamin), is a water-soluble vitamin. Pantothenic acid is an essential nutrient. Animals require pantothenic acid in order to synthesize coenzyme-A (CoA), as well as to synthesize and metabolize proteins, carbohydrates, and fats. The anion is called pantothenate.
Pantothenic acid is the amide between pantoic acid and β-alanine. Its name derives from the Greek pantothen, meaning "from everywhere", and small quantities of pantothenic acid are found in nearly every food, with high amounts in fortified whole-grain cereals, egg yolks, liver and dried mushrooms. It is commonly found as its alcohol analog, the provitamin panthenol (pantothenol), and as calcium pantothenate.
Pantothenic acid was discovered by Roger J. Williams in 1933.
Biological role
Only the dextrorotatory (D) isomer of pantothenic acid possesses biologic activity. The levorotatory (L) form may antagonize the effects of the dextrorotatory isomer.
Pantothenic acid is used in the synthesis of coenzyme A (CoA). Coenzyme A may act as an acyl group carrier to form acetyl-CoA and other related compounds; this is a way to transport carbon atoms within the cell. CoA is important in energy metabolism for pyruvate to enter the tricarboxylic acid cycle (TCA cycle) as acetyl-CoA, and for α-ketoglutarate to be transformed to succinyl-CoA in the cycle. CoA is also important in the biosynthesis of many important compounds such as fatty acids, cholesterol, and acetylcholine. CoA is incidentally also required in the formation of ACP, which is also required for fatty acid synthesis in addition to CoA.
Pantothenic acid in the form of CoA is also required for
acylation and acetylation, which, for example, are involved in signal
transduction and enzyme activation and deactivation, respectively.
Since pantothenic acid participates in a wide array of key biological roles, it is essential to all forms of life. As such, deficiencies in pantothenic acid may have numerous wide-ranging effects.
Sources
Dietary
Content of pantothenic acid varies among manufactured and natural foods, especially fortified ready-to-eat cereals, infant formulas, energy bars and dried foods. Major food sources of pantothenic acid are dried shiitake mushrooms, liver, kidney, egg yolks and sunflower seeds.
Whole grains are another source of the vitamin, but milling removes
much of the pantothenic acid, as it is found in the outer layers of
whole grains.
In animal feeds, the most important sources are alfalfa, cereal, fish
meal, peanut meal, molasses, mushrooms, rice, wheat bran, and yeasts.
Supplementation
The derivative of pantothenic acid, pantothenol (panthenol),
is a more stable form of the vitamin and is often used as a source of
the vitamin in multivitamin supplements. Another common supplemental
form of the vitamin is calcium pantothenate. Calcium pantothenate is
often used in dietary supplements because, as a salt, it is more stable
than pantothenic acid.
Dietary recommendations
The
U.S. Institute of Medicine (IOM) updated Estimated Average Requirements
(EARs) and Recommended Dietary Allowances (RDAs) for B vitamins in
1998. At that time there was not sufficient information to establish
EARs and RDAs for pantothenic acid. In instances such as this, the Board
sets Adequate Intakes (AIs), with the understanding that at some later
date, AIs will be replaced by more exact information. The current AI for
teens and adults ages 14 and up is 5 mg/day. AI for pregnancy is
6 mg/day. AI for lactation is 7 mg/day. For infants up to 12 months the
AI is 1.8 mg/day. For children ages 1–13 years the AI increases with age
from 2 to 4 mg/day. As for safety, the IOM sets Tolerable upper intake levels
(ULs) for vitamins and minerals when evidence is sufficient. In the
case of pantothenic acid there is no UL, as there is no human data for
adverse effects from high doses. Collectively the EARs, RDAs, AIs and
ULs are referred to as Dietary Reference Intakes (DRIs).
The European Food Safety Authority
(EFSA) refers to the collective set of information as Dietary Reference
Values, with Population Reference Intake (PRI) instead of RDA, and
Average Requirement instead of EAR. AI and UL are defined the same as in
the U.S. For women and men over age 11 the Adequate Intake (AI) is set
at 5 mg/day. AI for pregnancy is 5 mg/day, for lactation 7 mg/day. For
children ages 1–10 years the AI is 4 mg/day. These AIs are similar to
the U.S. AIs.[18]
The EFSA also reviewed the safety question and reached the same
conclusion as in United States - that there was not sufficient evidence
to set a UL for pantothenic acid.
For U.S. food and dietary supplement labeling purposes the amount
in a serving is expressed as a percent of Daily Value (%DV). For
pantothenic acid labeling purposes 100% of the Daily Value was 10 mg,
but as of May 27, 2016 it was revised to 5 mg to bring it into agreement
with the AI. A table of the old and new adult Daily Values is provided at Reference Daily Intake. Food and supplement companies have until January 1, 2020 to comply with the change.
| Age group | Age | Adequate intake |
|---|---|---|
| Infants | 0–6 months | 1.7 mg |
| Infants | 7–12 months | 1.8 mg |
| Children | 1–3 years | 2 mg |
| Children | 4–8 years | 3 mg |
| Children | 9–13 years | 4 mg |
| Adult men and women | 14+ years | 5 mg |
| Pregnant women | (vs. 5) | 6 mg |
| Breastfeeding women | (vs. 5) | 7 mg |
Absorption
When found in foods, most pantothenic acid is in the form of CoA or bound to acyl carrier protein
(ACP). For the intestinal cells to absorb this vitamin, it must be
converted into free pantothenic acid. Within the lumen of the
intestine, CoA and ACP are hydrolyzed into 4'-phosphopantetheine. The
4'-phosphopantetheine is then dephosphorylated into pantetheine. Pantetheinase, an intestinal enzyme, then hydrolyzes pantetheine into free pantothenic acid.
Free pantothenic acid is absorbed into intestinal cells via a saturable, sodium-dependent active transport system. At high levels of intake, when this mechanism is saturated, some pantothenic acid may also be absorbed via passive diffusion. As intake increases 10-fold, however, absorption rate decreases to 10%.
Deficiency
Pantothenic
acid deficiency is exceptionally rare and has not been thoroughly
studied. In the few cases where deficiency has been seen (victims of
starvation and limited volunteer trials), nearly all symptoms can be
reversed with the return of pantothenic acid.
Symptoms of deficiency are similar to other vitamin B deficiencies. There is impaired energy production, due to low CoA levels, which could cause symptoms of irritability, fatigue, and apathy. Acetylcholine synthesis is also impaired; therefore, neurological symptoms can also appear in deficiency; they include numbness, paresthesia, and muscle cramps. Deficiency in pantothenic acid can also cause hypoglycemia, or an increased sensitivity to insulin. Insulin receptors are acylated with palmitic acid when they do not want to bind with insulin. Therefore, more insulin will bind to receptors when acylation decreases, causing hypoglycemia. Additional symptoms could include restlessness, malaise, sleep disturbances, nausea, vomiting, and abdominal cramps. In a few rare circumstances, more serious (but reversible) conditions have been seen, such as adrenal insufficiency and hepatic encephalopathy.
Deficiency symptoms in other nonruminant animals include
disorders of the nervous, gastrointestinal, and immune systems, reduced
growth rate, decreased food intake, skin lesions and changes in hair
coat, and alterations in lipid and carbohydrate metabolism.
Toxicity
Toxicity of pantothenic acid is unlikely. In fact, no Tolerable Upper Level Intake (UL) has been established.
Large doses of the vitamin, when ingested, have no reported side
effects and massive doses (e.g., 10 g/day) may only yield mild diarrhea. There are also no adverse reactions known following parenteral (injected) or topical (skin) applications of the vitamin. Pantothenic acid, in an animal study, was shown to induce adrenal hyper-responsiveness to stress stimulation.
Research
Although pantothenic acid supplementation is under preliminary research for a variety of human diseases, there is insufficient evidence to date that it has any effect.
Ruminant nutrition
No dietary requirement for pantothenic acid has been established as synthesis of pantothenic acid by ruminal
microorganisms appears to be 20 to 30 times more than dietary amounts.
Net microbial synthesis of pantothenic acid in the rumen of steer
calves has been estimated to be 2.2 mg/kg of digestible organic matter
consumed per day. The degradation of dietary intake of pantothenic acid
is considered to be 78 percent. Supplementation of pantothenic acid at
5 to 10 times theoretic requirements did not improve performance of
feedlot cattle.

