Non-nucleoside reverse-transcriptase inhibitors (NNRTIs) are antiretroviral drugs used in the treatment of human immunodeficiency virus (HIV). NNRTIs inhibit reverse transcriptase (RT), an enzyme that controls the replication of the genetic material of HIV. RT is one of the most popular targets in the field of antiretroviral drug development.
Discovery and development of NNRTIs began in the late 1980s and in the end of 2009 four NNRTI had been approved by regulatory authorities and several others were undergoing clinical development. Drug resistance develops quickly if NNRTIs are administered as monotherapy and therefore NNRTIs are always given as part of combination therapy, the highly active antiretroviral therapy (HAART).
Discovery and development of NNRTIs began in the late 1980s and in the end of 2009 four NNRTI had been approved by regulatory authorities and several others were undergoing clinical development. Drug resistance develops quickly if NNRTIs are administered as monotherapy and therefore NNRTIs are always given as part of combination therapy, the highly active antiretroviral therapy (HAART).
History
Acquired immunodeficiency syndrome (AIDS) is a leading cause of death in the world. It was identified as a disease in 1981. Two years later the etiology agent for AIDS, the HIV was described. HIV is a retrovirus and has two major serotypes, HIV-1 and HIV-2. The pandemic mostly involves HIV-1 while HIV-2 has lower morbidity rate and is mainly restricted to western Africa.
In the year 2009 over 40 million people were infected worldwide with HIV and the number keeps on growing. The vast majority of infected individuals live in the developing countries.
HIV drugs do not cure HIV infection, but the treatment aims at improving the quality of patients´ lives and decreased mortality.
25 antiretroviral drugs were available in 2009 for the treatment
of HIV infection. The drugs belong to six different classes that act at
different targets. The most popular target in the field of
antiretroviral drug development is the HIV-1 reverse transcriptase (RT)
enzyme.
There are two classes of drugs that target the HIV-1 RT enzyme, nucleoside/nucleotide reverse-transcriptase inhibitors
(NRTIs/NtRTIs) and non-nucleoside reverse-transcriptase inhibitors
(NNRTIs). Drugs in these classes are important components of the HIV
combination therapy called highly active antiretroviral therapy, better
known as HAART.
In 1987, the first drug for the treatment of HIV infection was approved by the U.S. Food and Drug Administration (FDA). This was the NRTI called zidovudine.
In the late 1980s, during further development of NRTIs, the field of
NNRTIs discovery began. The development of NNRTIs improved quickly into
the 1990s and they soon became the third class of antiretroviral drugs,
following the protease inhibitors.
The NNRTIs are HIV-1 specific and have no activity against HIV-2 and other retroviruses. The first NNRTI, nevirapine was discovered by researchers at Boehringer Ingelheim and approved by the FDA in 1996. In the next two years two other NNRTIs were approved by the FDA, delavirdine in 1997 and efavirenz in 1998.
These three drugs are so-called first generation NNRTIs. The need for
NNRTIs with better resistance profile led to the development of the next
generation of NNRTIs. Researchers at Janssens Foundation and Tibotec discovered the first drug in this class, etravirine, which was approved by the FDA in 2008. The second drug in this class, rilpivirine,
was also discovered by Tibotec and was approved by the FDA in 2011. In
addition to these four NNRTIs several other are in clinical development.
The HIV-1 reverse transcriptase enzyme
Figure 1
This ribbon representation of the RT active domain (i.e. the p66
monomer) illustrates its hand-like structure, showing fingers (blue),
palm (pink) and thumb (green). The active site (red atoms), where DNA is
elongated, is in the palm region. Also shown is an NNRTI drug (yellow)
in the pocket where it binds.
Function
Reverse transcriptase (RT) is an enzyme that controls the replication of the genetic material of HIV and other retroviruses. The enzyme has two enzymatic functions. Firstly it acts as a polymerase where it transcribes the single-stranded RNA genome into single-stranded DNA
and subsequently builds a complementary strand of DNA. This provides a
DNA double helix which can be integrated in the host cell's chromosome. Secondly it has ribonuclease H (Rnase H) activity as it degrades the RNA strand of RNA-DNA intermediate that forms during viral DNA synthesis.
Structure
The HIV-1 RT is an asymmetric 1000-amino acid heterodimer composed of p66 (560 amino acids) and p51 subunits (440 amino acids). The p66 subunit has two domains, a polymerase and ribonuclease H. The polymerase domain contains four subdomains, which have been termed “fingers”, “palm”, “thumb” and “connection” and it is often compared to a right hand (figure 1). The role of the p66 subunit is to carry out the activity of RT whereas it contains the active sites of the enzyme. The p51 is believed to play mainly a structural role.
Binding and pharmacophore
Despite the chemical diversity of NNRTIs they all bind at the same site in the RT. The binding occurs allosterically in a hydrophobic pocket located approximately 10 Å from the catalytic site in the palm domain of the p66 subunit site of the enzyme. The NNRTI binding pocket (NNIBP) contains five aromatic (Tyr-181, Tyr-188, Phe-227 and Trp-229), six hydrophobic (Pro-59, Leu-100, Val-106, Val-179, Leu-234 and Pro-236) and five hydrophilic
(Lys-101, Lys-103, Ser-105, Asp-132 and Glu-224) amino acids that
belong to the p66 subunit and additional two amino acids (Ile-135 and
Glu-138) belonging to the p51 subunit. Each NNRTI interacts with different amino acid residues in the NNIBP.
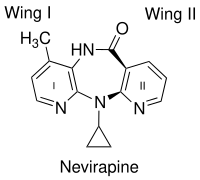
Figure 2 Chemical structure of nevirapine and the two wings.
An important factor in the binding of the first generation NNRTIs,
such as nevirapine, is the butterfly-like shape. Despite their chemical
diversity they assume very similar butterfly-like shape.
Two aromatic rings of NNRTIs conform within the enzyme to resemble the
wings of a butterfly (figure 2). The butterfly structure has a
hydrophilic centre as a ‘body’ and two hydrophobic moieties representing
the wings. Wing I is usually a heteroaromatic ring and wing II is a phenyl or allyl substituent. Wing I has a functional group
at one side of the ring which is capable of accepting and/or donating
hydrogen bonds with the main chain of the amino acids Lys-101 and
Lys-103. Wing II interacts through π-π interactions with a hydrophobic
pocket, formed in most part by the side chains of aromatic amino acids.
On the butterfly body a hydrophobic part fills a small pocket which is
mainly formed by the side chains of Lys-103, Val-106 and Val-179.
However many other NNRTIs have been found to bind to RT in different
modes. Second generation NNRTIs such as diarylpyrimidins (DAPYs), have a
horseshoe-like shape with two lateral hydrophobic wings and a pyrimidine ring which is the central polar part.
The NNIBP is elastic and the conformation depends on the size, specific chemical composition and binding mode of the NNRTI. The total structure of RT has segmental flexibility
that depends on the nature of the bound NNRTI. It's important for the
inhibitor to have flexibility to be able to bind in the modified pockets
of a mutant target. Inhibitor flexibility may not affect the
inhibitor-target interactions.
Mechanism of action
Figure 3
Non-nucleside reverse-transcriptase inhibitors (NNRTIs) inhibit the
reverse-transcriptase enzyme (RT) and therefore the replication of new
viruses
The NNRTIs act by binding non-competitively
to the RT enzyme (figure 3). The binding causes conformational change
in the three-dimensional structure of the enzyme and creates the NNIBP. Binding of NNRTI to HIV-1 RT makes the p66 thumb domain hyper extended because it induces rotamer conformation changes in amino acid residues Tyr-181 and Tyr-188.
This affects the catalytic activity of the enzyme and blocks the HIV-1
replication by inhibiting the polymerase active site of the RT's p66
subunit.
The global conformational change additionally destabilizes the enzyme
on its nucleic acid template and reduces its ability to bind
nucleotides. The transcription of the viral RNA is inhibited and therefore the replication rate of the virus reduces.
Although the exact molecular mechanism is still hypothetical this has
been demonstrated by multiple studies to be the primary mechanism of
action.
In addition to this proposed primary mechanism of action it has
been shown that the NNRTIs have other mechanisms of action and interfere
with various steps in the reverse transcriptase reaction. It has been suggested that the inhibition of reverse transcription by the NNRTIs may be due to effects on the RT Rnase H activity and/or template/primer binding. Some NNRTIs interfere with HIV-1 Gag-Pol polyprotein processing by inhibiting the late stage of HIV-1 replication.
It is important to gain profound understanding of the various
mechanism of action of the NNRTIs in order to develop next-generation
NNRTIs and for understanding the mechanism of drug resistance.
Drug discovery and design
The
development of effective anti-HIV drugs is difficult due to wide
variations in nucleotide and amino acid sequences. The perfect anti-HIV
drug chemical should be effective against drug resistance mutation.
Understanding the target RT enzyme and its structure, mechanism of drug
action and the consequence of drug resistance mutations provide useful
information which can be helpful to design more effective NNRTIs. The RT
enzyme can undergo change due to mutations that can disturb NNRTI
binding.
Discovery
The
first two classes of compounds that were identified as NNRTIs were the
1-(2-2-hydroxyethoxymethyl)-6-(phenylthio)thymine (HEPT) and
tetrahydroimidazo[4,5,1-jkj][1,4]benzodiazepin-2(1H)-one and -thione
(TIBO) compounds. The discovery of the TIBO compounds led to the
definition of the NNRTI class in the late 1980s
when they were unexpectedly found to inhibit RT. This finding initiated
researches on mechanism of action for these compounds. The HEPT
compounds were described before the TIBO compounds and were originally
believed to be NRTIs. Later it was discovered that they shared common
mechanism of action with the TIBO compounds.
Both the HEPT and TIBO compounds were first to be identified as highly
specific and potent HIV-1 RT inhibitors, not active against other RTs. These compounds do not interrupt the cellular or mitochondrial DNA synthesis. The specificity of the NNRTIs for HIV-1 is considered the hallmark of the NNRTI drug class.
Development
First generation NNRTIs
After
the discovery of HEPT and TIBO, compounds screening methods were used
to develop BI-RG-587, the first NNRTI commonly known as nevirapine. Like
HEPT and TIBO, nevirapine blocked viral RT activity by non-competitive
inhibition (with respect to dNTP binding). This reinforced the idea that
the new class of anti-HIV inhibitors was inhibiting the activity of RT
but not at the active site. Several molecular families of NNRTIs have
emerged following screening and evolution of many molecules.
Three NNRTI compounds of the first generation have been approved
by the FDA for treating HIV-1 infection. Nevirapine was approved in
1996, delavirdine in 1997 and efavirenz in 1998 (table 1). Two of these
drugs, nevirapine and efavirenz, are cornerstones of first line HAART
while delavirdine is hardly used nowadays.
The structure of these three drugs show the wide array of rings,
substituents, and bonds that allow activity against HIV-1 RT. This
diversity demonstrates why so many non-nucleosides have been synthesised
but doesn't explain why only three drugs have reached the market. The
main problem has been the potency of these compounds to develop
resistance.
Development from α-APA to ITU
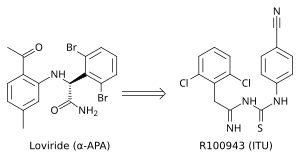
Figure 4 The development from α-APA to ITU
Crystal structure
analysis showed that the first generation NNRTIs (for example TIBO,
nevirapine and α-APA) bind HIV-1 RT in a “butterfly-like” conformation.
These first generation NNRTIs were vulnerable against the common
drug-resistance mutations like Tyr-181C and Tyr-188L/H. This triggered
the need for finding new and more effective NNRTIs. ITU
(imidoylthiourea), a promising series of NNRTIs emerged from α-APA
analogs (figure 4). The ITU compounds were obtained by extending the
linker that binds the aryl side groups of the α-APA. A potent ITU
compound, R100943, was obtained by an arrangement of the chemical
composition of the side groups based on structure-activity relationships
(SAR). A crystal structure of the HIV-1/R100943 complex demonstrated
that ITU compounds are more flexible than α-APA compound. The ITU
compounds showed distinct mode of binding where they bound with
"horseshoe" or "U" mode. The 2,6-dichlorophenyl part of R100943 which
corresponds chemically to the wing II 2,6-dibromophenyl part of the
α-APA occupied the wing I part in the NNIBP whereas the 4-cyanoanilino
part of R100943 occupies the wing II position in the NNIBP.
R100943 inhibited HIV-1 and was considerably effective against a
number of key NNRTI-resistant mutants like G190A mutation, which caused
high-level resistance to loviride (α-APA) and nevirapine. G190A
mutation was thought to cause resistance by occupying a part of the
binding pocket that would otherwise be filled by the linker part of the
butterfly shaped NNRTIs. R100943, in the horseshoe mode of binding, is
located at a distance of approximately 6.0 Å from G190. When compared
with nevirapine and loviride which bind in the butterfly shape the ITU derivatives
revealed improved activity against Tyr-181C and Tyr-188L mutants. A
structural study suggested that a potent TIBO compound could partly
supplement for the effects of the Tyr-181C mutation by moving itself in
the non-nucleoside inhibitor binding pocket (NNIBP) of the mutant RT. In
this context, R100943 has torsional freedom that enables the
conformational alternations of the NNRTI. This torsional freedom could
be used by the ITU derivate to bind to a mutated NNIBP and thus
compensating for the effects of a resistance mutation. Nevertheless, the
potency of R100943 against HIV-1 resistant mutants was not adequate for
it to be considered as an effective drug candidate. Additionally, the chemical stability of the imidoylthiourea part of the ITU derivative was not favorable for an oral drug.
Development from ITU to DATA
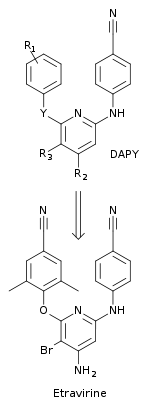
Figure 5 Chemical substitutions of the DAPY series were made to obtain the highly potent etravirine
Changes in the imidoylthiourea complexes led to the synthesis of a
new class of compounds, diaryltriazine (DATA). In these compounds, the
thiourea part of the ITU compounds was replaced by a triazine ring. The
DATA compounds were more potent than the ITU compounds against common
NNRTI resistant mutant strains. R106168, a prototype DATA compound, was
rather easy to synthesize. Multiple substitutions were made at different
positions on all of the three rings and on the linkers connecting the
rings. In the pocket, most of the DATA derivatives conformed a horseshoe
conformation. The two wings in R106168 (2,6-dichlorobenzyl and
4-cyanoanilino) occupied positions in the pocket similar to that of the
two wings of the derivatives of ITU. The central part of the DATA
compounds, in which the triazine ring replaced the thiourea group of ITU
derivatives, is positioned between the side chains of L100 and V179.
This removed a number of torstional degrees of freedom in the central
part while keeping the flexibility between the triazine ring and the
wings.
Chemical substitution or modification in the three-aromatic-ring
backbone of the DATA compounds had substantial effect on the activity.
R120393, a DATA analog, was designed with a chloroindole part in wing I
to expand interactions with the side chain of conserved W229 of the
polymerase primer grip loop. R120393 had similar effect as R106168
against most of the NNRTI-resistant mutants. The cloroindole part
interacted with the hydrophobic core of the pocket and influenced the
binding mode of the R120393 so it went deeper into the pocket compared
to the wing I position of other DATA analogs. Crystal structures showed
that the DATA compounds could bind the NNIBP in different conformations.
The capability to bind in multible modes made the NNRTIs stronger
against drug-resistance mutations. Variability between the inhibitors
could be seen when the chemical composition, size of wing I and the two
linker groups connecting the rings were altered. The potency of the
NNRTIs changed when the triazine nitrogen atoms were substituted with carbons.
Next generation NNRTIs
Researchers
used multi-disciplinary approach to design NNRTIs with better
resistance profile and an increased genetic barrier to the development
of resistance. A new class of compounds, diarylpyrimide (DAPY), were discovered with the replacement of the central triazine ring from the DATA compounds, with a pyrimidine.
This new class was more effective against drug resistant HIV-1 strains
than the corresponding DATA analogs. The replacement enabled
substitutions to the CH-group at the 5-position of the central aromatic
ring. One of the first DAPY compounds, dapivirine (with R1= 2,4,6-trimethylanilino, R2 = R3
= H and Y = NH) was found to be effective against drug-resistant HIV-1
strains. Systematic chemical substitutions were made at the R1, R2, R3
and Y positions to find new DAPY derivatives. This led to the discovery
of etravirine which has a bromine substitution at the 5-position (R3) of the pyrimidine ring (with R1 = 2,6-dimethyl-4-cyanoanilino, R2 = NH2 and Y = O) (figure 5).
Etravirine was discovered by researchers at the Jansen Research
Foundation and Tibotec and approved in 2008 by the FDA. It is used in
treatment-expirenced adult patients with HIV infection that is multidrug resistant in combination with other antiretroviral drugs.
Resistance
When
treating infection, whether bacterial or viral, there is always a risk
of the infectious agent to develop drug resistance. The treatment of HIV
infection is especially susceptible to drug resistance which is a
serious clinical concern in the chemotherapeutic treatment of the
infection. Drug resistant HIV-strains emerge if the virus is able to
replicate in the presence of the antiretroviral drugs.
NNRTI-resistant HIV-strains have the occurring mutations mainly
in and around the NNIBP affecting the NNRTI binding directly by altering
the size, shape and polarity on different areas of the pocket or by affecting, indirectly, the access to the pocket.
Those mutations are primarily noted in domains which span amino acids
98-108, 178-190 or 225-238 of the p66 subunit. The most frequent
mutations observed in viruses isolated from patients who have been on a
failing NNRTI containing chemotherapy are Lys-103N and Tyr-181C. NNRTI
resistance has been linked to over 40 amino acid substitutions in vitro and in vivo.
Antiretroviral drugs are never used in monotherapy due to rapid
resistance development. The highly active antiretroviral therapy (HAART)
was introduced in 1996. The treatment regimen combines three drugs from at least two different classes of antiretroviral drugs.
The advance of etravirine over other NNRTIs is that multiple
mutations are required for the development of drug resistance. The drug
has also shown activity against viruses with common NNRTI resistance
associated mutations and cross-resistance mutations.
Current status
Five
drugs in the class of NNRTIs have been approved by regulatory
authorities. These are the first generation NNRTIs nevirapine,
delavirdine and efavirenz and the next generation NNRTIs etravirine, and
rilpivirine. Several other NNRTIs underwent clinical development but were discontinued due to unfavourable pharmacokinetic, efficacy and/or safety factors.
Currently there are four other NNRTIs undergoing clinical development, IDX899, RDEA-428 and lersivirine (table 2).
Rilpivirine
Rilpivirine
is a DAPY compound like etravirine and was discovered when further
optimization within this family of NNRTIs was conducted. The resistance
profile and the genetic barrier to the development of resistance is
comparable to etravirine in vitro. The advantage of rilpivirine over etravirine is a better bioavailability and it is easier to formulate than etravirine. Etravirine has required extensive chemical formulation work due to poor solubility and bioavailability. Rilpivirine was undergoing phase III clinical trials in the end of 2009. Rilpivirine was approved by the FDA for HIV therapy in May 2011 under the brand name Edurant. Edurant is approved for treatment-naive patients with a viral load of 100,000 copies/mL or less at therapy initiation. Its recommended dosage is 25 mg orally once daily with a meal, in combination with other antiretrovirals.
It is contraindicated for use with proton pump inhibitors due to the
increased gastric pH causing decreased rilpivirine plasma
concentrations, potentially resulting in loss of virologic response and
possible resistance.
A fixed-dose drug combining rilpivirine with emtricitabine and
tenofovir disoproxil (TDF), was approved by the U.S. Food and Drug
Administration in August 2011 under the brand name Complera.
A newer fixed-dose drug also combining rilpivirine with emtricitabine
and tenofovir alafenamide (TAF) was approved in March 2016 under the
brand name Odefsey.
RDEA806
In 2007
a new family of triazole NNRTIs was presented by researchers from the
pharmaceutical company Ardea Biosciences. The selected candidate from
the screening executed was RDEA806 belonging to the family of triazoles. It has similar resistance profile against selected NNRTI resistant HIV-1 strains to other next generation NNRTIs. The candidate entered phase IIb clinical trials in the end of 2009, but no further trial have been initiated. Ardea was sold to AstraZeneca in 2012.
Fosdevirine (IDX899)
Fosdevirine
(also known as IDX899 and GSK-2248761) is another next generation NNRTI
developed by Idenix Pharmaceuticals and ViiV Healthcare. It belongs to
the family of 3-phosphoindoles. In vitro studies have shown comparable resistance profile to that of the other next generation NNRTIs.
In November 2009 the candidate entered phase II clinical trials, but
the trial and all further development was halted when 5 of 35 subjects
receiving fosdevirine experienced delayed-onset seizures.
Lersivirine (UK-453061)
Lersivirine belongs to the pyrazole family and is another next generation NNRTI in clinical trials developed by the pharmaceutical company ViiV Healthcare. The resistance profile is similar to that of other next generation NNRTIs. In the end of 2009 lersivirine was in phase IIb.
In February 2013, ViiV Healthcare announced a stop of the development program investigating lersivirine.