A drug interaction is a change in the action or side effects of a drug caused by concomitant administration with a food, beverage, supplement, or another drug.
There are many causes of drug interactions. For example, one drug may alter the pharmacokinetics of another. Alternatively, drug interactions may result from competition for a single receptor or signaling pathway.
The risk of a drug-drug interaction increases with the number of drugs used. Over a third (36%) of the elderly in the U.S. regularly use five or more medications or supplements, and 15% are at risk of a significant drug-drug interaction.
There are many causes of drug interactions. For example, one drug may alter the pharmacokinetics of another. Alternatively, drug interactions may result from competition for a single receptor or signaling pathway.
The risk of a drug-drug interaction increases with the number of drugs used. Over a third (36%) of the elderly in the U.S. regularly use five or more medications or supplements, and 15% are at risk of a significant drug-drug interaction.
Pharmacodynamic interactions
When
two drugs are used together, their effects can be additive (the result
is what you expect when you add together the effect of each drug taken
independently), synergistic (combining the drugs leads to a larger effect than expected), or antagonistic (combining the drugs leads to a smaller effect than expected).
There is sometimes confusion on whether drugs are synergistic or
additive, since the individual effects of each drug may vary from
patient to patient. A synergistic interaction may be beneficial for patients, but may also increase the risk of overdose.
Both synergy and antagonism can occur during different phases of
the interaction between a drug, and an organism. For example, when
synergy occurs at a cellular receptor level this is termed agonism, and the substances involved are termed agonists. On the other hand, in the case of antagonism, the substances involved are known as inverse agonists.
The different responses of a receptor to the action of a drug has
resulted in a number of classifications, such as "partial agonist",
"competitive agonist" etc. These concepts have fundamental applications
in the pharmacodynamics
of these interactions. The proliferation of existing classifications at
this level, along with the fact that the exact reaction mechanisms for
many drugs are not well-understood means that it is almost impossible to
offer a clear classification for these concepts. It is even possible
that many authors would misapply any given classification.
Direct interactions between drugs are also possible and may occur when two drugs are mixed prior to intravenous injection. For example, mixing thiopentone and suxamethonium in the same syringe can lead to the precipitation of thiopentone.
The change in an organism's response upon administration of a drug is an important factor in pharmacodynamic
interactions. These changes are extraordinarily difficult to classify
given the wide variety of modes of action that exist, and the fact that
many drugs can cause their effect through a number of different
mechanisms. This wide diversity also means that, in all but the most
obvious cases it is important to investigate, and understand these
mechanisms. The well-founded suspicion exists that there are more
unknown interactions than known ones.
Effects
of the competitive inhibition of an agonist by increases in the
concentration of an antagonist. A drugs potency can be affected (the
response curve shifted to the right) by the presence of an antagonistic
interaction.pA2 known as the Schild representation, a
mathematical model of the agonist:antagonist relationship or vice versa.
NB: the x-axis is incorrectly labelled and should reflect the agonist concentration, not antagonist concentration.
Pharmacodynamic interactions can occur on:
- Pharmacological receptors: Receptor
interactions are the most easily defined, but they are also the most
common. From a pharmacodynamic perspective, two drugs can be considered
to be:
- Homodynamic, if they act on the same receptor. They, in turn can be:
- Pure agonists, if they bind to the main locus of the receptor, causing a similar effect to that of the main drug.
- Partial agonists if, on binding to one of the receptor's secondary sites, they have the same effect as the main drug, but with a lower intensity.
- Antagonists, if they bind directly to the receptor's main locus but their effect is opposite to that of the main drug. These include:
- Competitive antagonists, if they compete with the main drug to bind with the receptor. The amount of antagonist or main drug that binds with the receptor will depend on the concentrations of each one in the plasma.
- Uncompetitive antagonists, when the antagonist binds to the receptor irreversibly and is not released until the receptor is saturated. In principle the quantity of antagonist and agonist that binds to the receptor will depend on their concentrations. However, the presence of the antagonist will cause the main drug to be released from the receptor regardless of the main drug's concentration, therefore all the receptors will eventually become occupied by the antagonist.
- Heterodynamic competitors, if they act on distinct receptors.
- Homodynamic, if they act on the same receptor. They, in turn can be:
- Signal transduction mechanisms: these are molecular processes that commence after the interaction of the drug with the receptor. For example, it is known that hypoglycaemia (low blood glucose) in an organism produces a release of catecholamines, which trigger compensation mechanisms thereby increasing blood glucose levels. The release of catecholamines also triggers a series of symptoms, which allows the organism to recognise what is happening and which act as a stimulant for preventative action (eating sugars). Should a patient be taking a drug such as insulin, which reduces glycaemia, and also be taking another drug such as certain beta-blockers for heart disease, then the beta-blockers will act to block the adrenaline receptors. This will block the reaction triggered by the catecholamines should a hypoglycaemic episode occur. Therefore, the body will not adopt corrective mechanisms and there will be an increased risk of a serious reaction resulting from the ingestion of both drugs at the same time.
- Antagonic physiological systems: Imagine a drug A that acts on a certain organ. This effect will increase with increasing concentrations of physiological substance S in the organism. Now imagine a drug B that acts on another organ, which increases the amount of substance S. If both drugs are taken simultaneously it is possible that drug A could cause an adverse reaction in the organism as its effect will be indirectly increased by the action of drug B. An actual example of this interaction is found in the concomitant use of digoxin and furosemide. The former acts on cardiac fibres and its effect is increased if there are low levels of potassium (K) in blood plasma. Furosemide is a diuretic that lowers arterial tension but favours the loss of K+. This could lead to hypokalemia (low levels of potassium in the blood), which could increase the toxicity of digoxin.
Pharmacokinetic interactions
Modifications
in the effect of a drug are caused by differences in the absorption,
transport, distribution, metabolism or excretion of one or both of the
drugs compared with the expected behavior of each drug when taken
individually. These changes are basically modifications in the
concentration of the drugs. In this respect, two drugs can be homergic
if they have the same effect in the organism and heterergic if their
effects are different.
Absorption interactions
Changes in motility
Some
drugs, such as the prokinetic agents increase the speed with which a
substance passes through the intestines. If a drug is present in the
digestive tract's absorption zone for less time its blood concentration
will decrease. The opposite will occur with drugs that decrease
intestinal motility.
- pH: Drugs can be present in either ionised or non-ionised form, depending on their pKa (pH at which the drug reaches equilibrium between its ionised and non-ionised form). The non-ionized forms of drugs are usually easier to absorb, because they will not be repelled by the lipidic bylayer of the cell, most of them can be absorbed by passive diffusion, unless they are too big or too polarized (like glucose or vancomycin), in which case they may have or not have specific and non specific transporters distributed on the entire intestine internal surface, that carries drugs inside the body. Obviously increasing the absorption of a drug will increase its bioavailability, so, changing the drug's state between ionized or not, can be useful or not for certain drugs.
Certain drugs require an acid stomach pH
for absorption. Others require the basic pH of the intestines. Any
modification in the pH could change this absorption. In the case of the antacids, an increase in pH can inhibit the absorption of other drugs such as zalcitabine (absorption can be decreased by 25%), tipranavir (25%) and amprenavir (up to 35%). However, this occurs less often than an increase in pH causes an increase in absorption. Such as occurs when cimetidine is taken with didanosine. In this case a gap of two to four hours between taking the two drugs is usually sufficient to avoid the interaction.
- Drug solubility: The absorption of some drugs can be drastically reduced if they are administered together with food with a high fat content. This is the case for oral anticoagulants and avocado.
- Formation of non-absorbable complexes:
- Chelation: The presence of di- or trivalent cations can cause the chelation of certain drugs, making them harder to absorb. This interaction frequently occurs between drugs such as tetracycline or the fluoroquinolones and dairy products (due to the presence of Ca++).
- Binding with proteins. Some drugs such as sucralfate binds to proteins, especially if they have a high bioavailability. For this reason its administration is contraindicated in enteral feeding.
- Finally, another possibility is that the drug is retained in the intestinal lumen forming large complexes that impede its absorption. This can occur with cholestyramine if it is associated with sulfamethoxazol, thyroxine, warfarin or digoxin.
- Acting on the P-glycoprotein of the enterocytes: This appears to be one of the mechanisms promoted by the consumption of grapefruit juice in increasing the bioavailability of various drugs, regardless of its demonstrated inhibitory activity on first pass metabolism.
Transport and distribution interactions
The
main interaction mechanism is competition for plasma protein transport.
In these cases the drug that arrives first binds with the plasma
protein, leaving the other drug dissolved in the plasma, which modifies
its concentration. The organism has mechanisms to counteract these
situations (by, for example, increasing plasma clearance),
which means that they are not usually clinically relevant. However,
these situations should be taken into account if other associated
problems are present such as when the method of excretion is affected.
Metabolism interactions
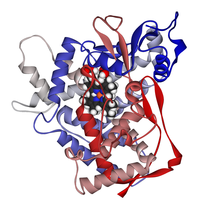
Diagram of cytochrome P450 isoenzyme 2C9 with the haem group in the centre of the enzyme.
Many drug interactions are due to alterations in drug metabolism. Further, human drug-metabolizing enzymes are typically activated through the engagement of nuclear receptors. One notable system involved in metabolic drug interactions is the enzyme system comprising the cytochrome P450 oxidases.
CYP450
Cytochrome P450 is a very large family of haemoproteins (hemoproteins) that are characterized by their enzymatic activity and their role in the metabolism of a large number of drugs.
Of the various families that are present in human beings the most
interesting in this respect are the 1, 2 and 3, and the most important
enzymes are CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4.
The majority of the enzymes are also involved in the metabolism of endogenous substances, such as steroids or sex hormones,
which is also important should there be interference with these
substances. As a result of these interactions the function of the
enzymes can either be stimulated (enzyme induction) or inhibited.
Enzymatic inhibition
If
drug A is metabolized by a cytochrome P450 enzyme and drug B inhibits
or decreases the enzyme's activity, then drug A will remain with high
levels in the plasma for longer as its inactivation is slower. As a
result, enzymatic inhibition will cause an increase in the drug's
effect. This can cause a wide range of adverse reactions.
It is possible that this can occasionally lead to a paradoxical
situation, where the enzymatic inhibition causes a decrease in the
drug's effect: if the metabolism of drug A gives rise to product A2, which actually produces the effect of the drug. If the metabolism of drug A is inhibited by drug B the concentration of A2 that is present in the blood will decrease, as will the final effect of the drug.
Enzymatic induction
If
drug A is metabolized by a cytochrome P450 enzyme and drug B induces or
increases the enzyme's activity, then blood plasma concentrations of
drug A will quickly fall as its inactivation will take place more
rapidly. As a result, enzymatic induction will cause a decrease in the
drug's effect.
As in the previous case, it is possible to find paradoxical
situations where an active metabolite causes the drug's effect. In this
case the increase in active metabolite A2 (following the previous example) produces an increase in the drug's effect.
It can often occur that a patient is taking two drugs that are
enzymatic inductors, one inductor and the other inhibitor or both
inhibitors, which greatly complicates the control of an individual's
medication and the avoidance of possible adverse reactions.
An example of this is shown in the following table for the CYP1A2
enzyme, which is the most common enzyme found in the human liver. The
table shows the substrates (drugs metabolized by this enzyme) and the
inductors and inhibitors of its activity:
| Substrates | Inhibitors | Inductors |
|---|---|---|
Enzyme CYP3A4
is the enzyme that the greatest number of drugs use as a substrate.
Over 100 drugs depend on its metabolism for their activity and many
others act on the enzyme as inductors or inhibitors.
Some foods also act as inductors or inhibitors of enzymatic activity. The following table shows the most common:
| Food | Mechanism | Drugs affected |
|---|---|---|
| Enzymatic inductor | Acenocoumarol, warfarin | |
| Grapefruit juice | Enzymatic inhibition | |
| Soya | Enzymatic inhibition | Clozapine, haloperidol, olanzapine, caffeine, NSAIDs, phenytoin, zafirlukast, warfarin |
| Garlic | Increases antiplatelet activity | |
| Ginseng | To be determined | Warfarin, heparin, aspirin and NSAIDs |
| Ginkgo biloba | Strong inhibitor of platelet aggregation factor | Warfarin, aspirin and NSAIDs |
| Hypericum perforatum (St John's wort) | Enzymatic inductor (CYP450) | Warfarin, digoxin, theophylline, cyclosporine, phenytoin and antiretrovirals |
| Ephedra | Receptor level agonist | MAOI, central nervous system stimulants, alkaloids ergotamines and xanthines |
| Kava (Piper methysticum) | Unknown | Levodopa |
| Ginger | Inhibits thromboxane synthetase (in vitro) | Anticoagulants |
| Chamomile | Unknown | Benzodiazepines, barbiturates and opioids |
| Hawthorn | Unknown | Beta-adrenergic antagonists, cisapride, digoxin, quinidine |
Grapefruit juice can act as an enzyme inhibitor.
Any study of pharmacological interactions between particular
medicines should also discuss the likely interactions of some medicinal
plants. The effects caused by medicinal plants should be considered in
the same way as those of medicines as their interaction with the
organism gives rise to a pharmacological response. Other drugs can
modify this response and also the plants can give rise to changes in the
effects of other active ingredients.
There is little data available regarding interactions involving medicinal plants for the following reasons:
St John's wort can act as an enzyme inductor.
- False sense of security regarding medicinal plants. The interaction between a medicinal plant and a drug is usually overlooked due to a belief in the "safety of medicinal plants."
- Variability of composition, both qualitative and quantitative. The composition of a plant-based drug is often subject to wide variations due to a number of factors such as seasonal differences in concentrations, soil type, climatic changes or the existence of different varieties or chemical races within the same plant species that have variable compositions of the active ingredient. On occasion, an interaction can be due to just one active ingredient, but this can be absent in some chemical varieties or it can be present in low concentrations, which will not cause an interaction. Counter interactions can even occur. This occurs, for instance, with ginseng, the Panax ginseng variety increases the Prothrombin time, while the Panax quinquefolius variety decreases it.
- Absence of use in at-risk groups, such as hospitalized and polypharmacy patients, who tend to have the majority of drug interactions.
- Limited consumption of medicinal plants has given rise to a lack of interest in this area.
They are usually included in the category of foods as they are
usually taken as a tea or food supplement. However, medicinal plants are
increasingly being taken in a manner more often associated with
conventional medicines: pills, tablets, capsules, etc.
Excretion interactions
Renal excretion
Human kidney nephron.
Only the free fraction of a drug that is dissolved in the blood plasma can be removed through the kidney.
Therefore, drugs that are tightly bound to proteins are not available
for renal excretion, as long as they are not metabolized when they may
be eliminated as metabolites.
Creatinine clearance
is used as a measure of kidney functioning but it is only useful in
cases where the drug is excreted in an unaltered form in the urine. The
excretion of drugs from the kidney's nephrons has the same properties as
that of any other organic solute: passive filtration, reabsorption and
active secretion. In the latter phase the secretion of drugs is an
active process that is subject to conditions relating to the
saturability of the transported molecule and competition between
substrates. Therefore, these are key sites where interactions between
drugs could occur.
Filtration depends on a number of factors including the pH of the urine, it having been shown that the drugs that act as weak bases are increasingly excreted as the pH of the urine becomes more acidic, and the inverse is true for weak acids.
This mechanism is of great use when treating intoxications (by making
the urine more acidic or more alkali) and it is also used by some drugs
and herbal products to produce their interactive effect.
| Weak acids | Weak bases |
|---|---|
Bile excretion
Bile
excretion is different from kidney excretion as it always involves
energy expenditure in active transport across the epithelium of the bile
duct against a concentration gradient.
This transport system can also be saturated if the plasma
concentrations of the drug are high. Bile excretion of drugs mainly
takes place where their molecular weight is greater than 300 and they contain both polar and lipophilic groups. The glucuronidation
of the drug in the kidney also facilitates bile excretion. Substances
with similar physicochemical properties can block the receptor, which is
important in assessing interactions. A drug excreted in the bile duct
can occasionally be reabsorbed by the intestines (in the enterohepatic
circuit), which can also lead to interactions with other drugs.
Herb-drug interactions
Herb-drug interactions are drug interactions that occur between herbal medicines and conventional drugs.
These types of interactions may be more common than drug-drug
interactions because herbal medicines often contain multiple
pharmacologically active ingredients, while conventional drugs typically
contain only one. Some such interactions are clinically significant, although most herbal remedies are not associated with drug interactions causing serious consequences. Most herb-drug interactions are moderate in severity. The most commonly implicated conventional drugs in herb-drug interactions are warfarin, insulin, aspirin, digoxin, and ticlopidine, due to their narrow therapeutic indices. The most commonly implicated herbs involved in such interactions are those containing St. John’s Wort, magnesium, calcium, iron, or ginkgo.
Examples
Examples of herb-drug interactions include, but are not limited to:
- St. John's wort affects the clearance of numerous drugs, including cyclosporin, SSRI antidepressants, digoxin, indinavir, and phenprocoumon. It may also interact with the anti-cancer drugs irinotecan and imatinib.
- Salvia miltiorrhiza may enhance anticoagulation and bleeding among people taking warfarin.
- Allium sativum has been found to decrease the plasma concentration of saquinavir, and may cause hypoglycemia when taken with chlorpropamide.
- Ginkgo biloba can cause bleeding when combined with warfarin or aspirin.
- Concomitant ephedra and caffeine use has been reported to, in rare cases, cause fatalities.
Mechanisms
The mechanisms underlying most herb-drug interactions are not fully understood. Interactions between herbal medicines and anticancer drugs typically involve enzymes that metabolize cytochrome P450. For example, St. John's Wort has been shown to induce CYP3A4 and P-glycoprotein in vitro and in vivo.
Underlying factors
It
is possible to take advantage of positive drug interactions. However,
the negative interactions are usually of more interest because of their
pathological significance, and also because they are often unexpected,
and may even go undiagnosed. By studying the conditions that favor the
appearance of interactions, it should be possible to prevent them, or at
least diagnose them in time. The factors or conditions that predispose
the appearance of interactions include:
- Old age: factors relating to how human physiology changes with age may affect the interaction of drugs. For example, liver metabolism, kidney function, nerve transmission or the functioning of bone marrow all decrease with age. In addition, in old age there is a sensory decrease that increases the chances of errors being made in the administration of drugs.
- Polypharmacy: The use of multiple drugs by a single patient, to treat one or more ailments. The more drugs a patient takes the more likely it will be that some of them will interact.
- Genetic factors: Genes synthesize enzymes that metabolize drugs. Some races have genotypic variations that could decrease or increase the activity of these enzymes. The consequence of this would, on occasions, be a greater predisposition towards drug interactions and therefore a greater predisposition for adverse effects to occur. This is seen in genotype variations in the isozymes of cytochrome P450.
- Hepatic or renal diseases: The blood concentrations of drugs that are metabolized in the liver and/or eliminated by the kidneys may be altered if either of these organs is not functioning correctly. If this is the case an increase in blood concentration is normally seen.
- Serious diseases that could worsen if the dose of the medicine is reduced.
- Drug dependent factors:
- Narrow therapeutic index: Where the difference between the effective dose and the toxic dose is small. The drug digoxin is an example of this type of drug.
- Steep dose-response curve: Small changes in the dosage of a drug produce large changes in the drug's concentration in the patient's blood plasma.
- Saturable hepatic metabolism: In addition to dose effects the capacity to metabolize the drug is greatly decreased
Epidemiology
Among US adults older than 55, 4% are taking medication and or supplements that put them at risk of a major drug interaction. Potential drug-drug interactions have increased over time and are more common in the low educated elderly even after controlling for age, sex, place of residence, and comorbidity.