A
first-generation e-cigarette that resembles a tobacco cigarette. The
battery portion of the e-cigarette can be disconnected and recharged
using the USB power charger.
Various
types of e-cigarettes, including a disposable e-cigarette, a
rechargeable e-cigarette, a medium-size tank device, large-size tank
devices, an e-cigar, and an e-pipe.
An electronic cigarette, also known as e-cigarette among other names, is a handheld battery-powered vaporizer that simulates smoking and provides some of the behavioral aspects of smoking, including the hand-to-mouth action of smoking, but without burning tobacco. Using an e-cigarette is known as "vaping" and the user is referred to as a "vaper." Instead of cigarette smoke, the user inhales an aerosol, commonly called vapor. E-cigarettes typically have a heating element that atomizes a liquid solution called e-liquid. E-cigarettes are automatically activated by taking a puff; others turn on manually by pressing a button. Some e-cigarettes look like traditional cigarettes, but they come in many variations. Most versions are reusable, though some are disposable. There are first-generation, second-generation, third-generation, and fourth-generation devices. There is also pod mod devices that use nicotine in the form of a protonated nicotine, rather than free-base nicotine found in earlier generations. E-liquids usually contain propylene glycol, glycerin, nicotine, flavorings, additives, and differing amounts of contaminants. E-liquids are also sold without propylene glycol, nicotine, or flavors.
The benefits and the health risks of e-cigarettes are uncertain. There is tentative evidence they may help people quit smoking, although they have not been proven to be more effective than smoking cessation medicine.
There is concern with the possibility that non-smokers and children may
start nicotine use with e-cigarettes at a rate higher than anticipated
than if they were never created. Following the possibility of nicotine addiction from e-cigarette use, there is concern children may start smoking cigarettes. Youth who use e-cigarettes are more likely to go on to smoke cigarettes. Their part in tobacco harm reduction is unclear, while another review found they appear to have the potential to lower tobacco-related death and disease. Regulated US Food and Drug Administration (US FDA) nicotine replacement products may be safer than e-cigarettes, but e-cigarettes are generally seen as safer than combusted tobacco products. The risk of early death is anticipated to be similar to that of smokeless tobacco. The long-term effects of e-cigarette use are unknown. The risk from serious adverse events was reported in 2016 to be low. Less serious adverse effects include abdominal pain, headache, blurry vision, throat and mouth irritation, vomiting, nausea, and coughing. Nicotine itself is associated with some health harms. In 2019, an outbreak of severe lung illness across multiple states in the US was linked to vaping.
E-cigarettes create vapor made of fine and ultrafine particles of particulate matter, which have been found to contain propylene glycol, glycerin, nicotine, flavors, small amounts of toxicants, carcinogens, and heavy metals, as well as metal nanoparticles, and other substances. Its exact composition varies across and within manufacturers,
and depends on the contents of the liquid, the physical and electrical
design of the device, and user behavior, among other factors. E-cigarette vapor potentially contains harmful chemicals not found in tobacco smoke. E-cigarette vapor contains fewer toxic chemicals, and lower concentrations of potential toxic chemicals than cigarette smoke. The vapor is probably much less harmful to users and bystanders than cigarette smoke, although concern exists that the exhaled vapor may be inhaled by non-users, particularly indoors.
Since their entrance to the market in 2003, global use has risen exponentially. In a 2014 survey, about 13% of American high school students reported using them at least once in the previous month, and in 2015 around 10% of American adults were users. In the UK, users have increased from 700,000 in 2012 to 2.6 million in 2015. About 60% of UK users are smokers and about 40% are ex-smokers, while use among never-smokers in the UK is negligible. Most still use traditional cigarettes, raising concern that dual use may "delay or deter quitting". Most peoples' reason for using e-cigarettes involve trying to quit smoking, though a large proportion use them recreationally. It is commonly stated that the modern e-cigarette was invented in 2003 by Chinese pharmacist Hon Lik, but tobacco companies had been developing nicotine aerosol generation devices since as early as 1963. As of 2018, 95% of e-cigarettes were made in China. Because of overlap with tobacco laws and medical drug policies, e-cigarette legislation is being debated in many countries. The revised EU Tobacco Products Directive came into effect in May 2016, providing stricter regulations for e-cigarettes. As of August 2016, the US FDA extended its regulatory power to include e-cigarettes. Large tobacco companies have greatly increased their marketing efforts. As of 2014, there were 466 brands of e-cigarettes, with global sales of around $7 billion.
Use
Frequency
Since their introduction to the market in 2003, global usage of e-cigarettes has risen exponentially. By 2013, there were several million users globally.
Awareness and use of e-cigarettes greatly increased over the few years
leading up to 2014, particularly among young people and women in some
parts of the world. A 2013 four-country survey found there was generally greater awareness among white adult smokers compared with non-white ones. Vaping is increasing in the majority of high-income countries. E-cigarette use in the US and Europe is higher than in other countries, except for China which has the greatest number of e-cigarette users. Growth in the US had reportedly slowed in 2015, lowering market forecasts for 2016. Growth in the UK as of January 2018 had reportedly slowed since 2013.
The growing frequency of e-cigarette use may be due to heavy promotion
in youth-driven media channels, their low cost, and the misbelief that
e-cigarettes are safer than traditional cigarettes, according to a 2016
review.
Surveys in 2010 and 2011 suggested that adults with higher
incomes were more likely to have heard of e-cigarettes, but those with
lower incomes may have been more likely to try them. Most users had a history of smoking regular cigarettes, while results by race were mixed. At least 52% of smokers or ex-smokers have used an e-cigarette. Of smokers who have, less than 15% become everyday e-cigarette users. Though e-cigarette use among those who have never smoked is very low, it continues to rise. Daily vapers are typically recent former smokers. E-cigarettes are commonly used among non-smokers. This includes young adult non-smokers.
Vaping is the largest among adults between 18 and 24 years of age, and
use is the largest among adults who do not have a high school diploma. Young adults who vape but do not smoke are more than twice as likely to intend to try smoking than their peers who do not vape. A worldwide survey of e-cigarette users conducted in 2014 found that only 3.5% of respondents used liquid without nicotine.
Greater than 10 million people vape daily, as of 2018. Everyday use is common among e-cigarette users. E-cigarette users mostly keep smoking traditional cigarettes. Adults often vape to replace tobacco. Most vapers still use nicotine liquids after stopping smoking for several months. Most e-cigarette users are middle-aged men who also smoke traditional cigarettes, either to help them quit or for recreational use. Older people are more likely to vape for quitting smoking than younger people. Men were found to use higher nicotine doses, compared with women who were vaping.
Gender was found to be a predictor of current e-cigarette use with
males being significantly more likely to declare having already tried it
than females. Among young adults e-cigarette use is not regularly associated with trying to quit smoking. The research indicates that the most common way people try to quit smoking in the UK is with e-cigarettes.
Dual use of e-cigarettes and traditional tobacco is still a definite concern. Dual use of e-cigarettes with cigarettes is the most frequent pattern. One-time e-cigarette use seems to be higher in people with greater levels of educational achievement. Women smokers who are poorer and did not finish high school are more likely to have tried vaping at least once.
Vocational school career, lower school performance, being out of
school, and studying at a disadvantaged school have been shown to be
associated with both e-cigarette ever use and e-cigarette daily use. Vaping is increasing among people with cancer who are frequent smokers.
Gateway theory
In the context of drugs, the gateway hypothesis predicts that the use of less deleterious drugs can lead to a future risk of using more dangerous hard drugs or crime. There is wide concern that vaping may be a "gateway" to smoking. Vaping may also act as a gateway to illicit drug use (recreational use of illegal drugs), is an area of concern. Studies indicate vaping serves as a gateway to traditional cigarettes and cannabis use. Nicotine is a gateway to opioid addiction, as nicotine lowers the threshold for addiction to other agents.
Under the common liability model, some have suggested that any
favorable relation between vaping and starting smoking is a result of
common risk factors. This includes impulsive and sensation seeking personality types or exposure to people who are sympathetic with smoking and relatives. A 2014 review using animal models found that nicotine exposure may increase the likelihood to using other drugs, independent of factors associated with a common liability.
The gateway theory, in relation to using nicotine, has also been used
as a way to propose that using tobacco-free nicotine is probably going
to lead to using nicotine via tobacco smoking, and therefore that vaping
by non-smokers, and especially by children, may result in smoking
independent of other factors associated with starting smoking.
Some see the gateway model as a way to illustrate the potential
risk-heightening effect of vaping and going on to use combusted tobacco
products.
There is concern regarding that the accessibility of e-liquid flavors could lead to using additional tobacco products among non-smokers. It is argued to implement the precautionary principle because vaping by non-smokers may lead to smoking.
There is a concern with the possibility that non-smokers as well as
children may start nicotine use with e-cigarettes at a rate higher than
anticipated than if they were never created.
In certain cases, e-cigarettes might increase the likelihood of being
exposed to nicotine itself, especially for never-nicotine users who
start using nicotine products only as a result of these devices.
A 2015 review concluded that "Nicotine acts as a gateway drug on the
brain, and this effect is likely to occur whether the exposure is from
smoking tobacco, passive tobacco smoke or e-cigarettes."
Because those with mental illness are highly predisposed to nicotine
addiction, those who try e-cigarettes may be more likely to become
dependent, raising concerns about facilitating a transition to
combustible tobacco use. Even if an e-cigarette contains no nicotine, the user mimics the actions of smoking. This may renormalize tobacco use in the general public. Normalization of e-cigarette use may lead former cigarette smokers to begin using them, thereby reinstating their nicotine dependence and fostering a return to tobacco use. There is a possible risk of re-normalizing of tobacco use in areas where smoking is banned. Government intervention is recommended to keep children safe from the re-normalizing of tobacco, according to a 2017 review.
The "catalyst model" suggests that vaping may proliferate smoking
in minors by sensitizing minors to nicotine with the use of a type of
nicotine that is more pleasing and without the negative attributes of
regular cigarettes.
A 2016 review, based on the catalyst model, "indicate that the
perceived health risks, specific product characteristics (such as taste,
price and inconspicuous use), and higher levels of acceptance among
peers and others potentially make e-cigarettes initially more attractive
to adolescents than tobacco cigarettes. Later, increasing familiarity
with nicotine could lead to the reevaluation of both electronic and
tobacco cigarettes and subsequently to a potential transition to tobacco
smoking."
Pregnancy
E-cigarette use was also rising among women, including women of childbearing age as of 2014, but the rate of use during pregnancy is unknown. Many woman still vape during pregnancy because of their perceived safety in comparison with tobacco.
In one of the few studies identified, a 2015 survey of 316 pregnant
women in a Maryland clinic found that the majority had heard of
e-cigarettes, 13% had ever used them, and 0.6% were current daily users.
These findings are of concern because the dose of nicotine delivered by
e-cigarettes can be as high or higher than that delivered by
traditional cigarettes. The rate of e-cigarette use among pregnant adolescents is unknown.
Youth
Today, more high school students use e-cigarettes than regular cigarettes. The use of e-cigarettes is higher among high school students than adults.
The prevalence of vaping among adolescents is increasing worldwide. There is substantial variability in vaping in youth worldwide across countries.
Over the years leading up to 2017 vaping among adolescents has grown
every year since these devices were first introduced to the market. There appears to be an increase of one-time e-cigarette use among young people worldwide. The frequency of vaping in youth is low. The result of youth e-cigarette use leading to smoking is unclear. Most e-cigarette users among youth have never smoked. Many youth who use e-cigarettes also smoke traditional cigarettes.
Some youths who have tried an e-cigarette have never used a traditional
cigarette; indicating e-cigarettes may be a starting point for nicotine
use. Adolescents who would have not been using nicotine products to begin with are vaping. Twice as many youth vaped in 2014 than also used traditional cigarettes. Vaping seems to be a gateway to using traditional cigarettes in adolescents. Youth who use e-cigarettes are more likely to go on to use traditional cigarettes. The evidence suggests that young people who vape are also at greater risk for subsequent long-term tobacco use.
E-cigarettes are expanding the nicotine market by attracting low-risk
youth who would be unlikely to initiate nicotine use with traditional
cigarettes.
Data from a longitudinal cohort study of children with alcoholic
parents found that adolescents (both middle and late adolescence) who
used cigarettes, marijuana, or alcohol were significantly more likely to
have ever used e-cigarettes. Adolescents were more likely to initiate vaping through flavored e-cigarettes. Among youth who have ever tried an e-cigarette, a majority used a flavored product the first time they tried an e-cigarette. There is a greater likelihood of past or present and later cannabis use among youth and young adults who have vaped.
2014 Centers for Disease Control and Prevention press release about e-cigarettes.
Most youth are not vaping to help them quit tobacco. Adolescent vaping is unlikely to be associated with trying to reduce or quit tobacco. Adolescents who vape but do not smoke are more than twice as likely to intend to try smoking than their peers who do not vape.
Vaping is correlated with a higher occurrence of cigarette smoking
among adolescents, even in those who otherwise may not have been
interested in smoking. Adolescence experimenting with e-cigarettes appears to encourage continued use of traditional cigarettes. A 2015 study found minors had little resistance to buying e-cigarettes online.
An emerging concern is that nicotine, fruit flavors, and other e-liquid
additives could incite teenagers and children to start using
traditional cigarettes. Teenagers may not admit using e-cigarettes, but use, for instance, a hookah pen. As a result, self-reporting may be lower in surveys. Experts suggest that candy-like flavors could lead youths to experiment with vaping. E-cigarette advertisements seen by youth could increase the likelihood among youths to experiment with vaping.
A 2016 review found "The reasons for the increasing use of e-cigarettes
by minors (persons between 12 and 17 years of age) may include robust
marketing and advertising campaigns that showcase celebrities, popular
activities, evocative images, and appealing flavors, such as cotton
candy."
A 2014 survey stated that vapers may have less social and behavioral
stigma than cigarette smokers, causing concern that vaping products are
enticing youth who may not under other circumstances have used these
products. The frequency of vaping is higher in adolescent with asthma than in adolescent who do not have asthma. Boys had experimented more often than girls.
Motivation
There are varied reasons for e-cigarette use. Most users' motivation is related to trying to quit smoking, but a large proportion of use is recreational.
Adults cite predominantly three reasons for trying and using
e-cigarettes: as an aid to smoking cessation, a belief that they are a
safer alternative to traditional cigarettes, and as a way to
conveniently get around smoke-free laws. Some users vape for the enjoyment of the activity. Many e-cigarette users use them because they believe they are safer than traditional cigarettes. People who think they pose less risk than cigarette smoking are more likely to vape.
A 2017 report found that smokers who previously vaped and quit though
continued smoking, 51.5% believed that vaping is less risky than smoking In contrast, 90% of former-smokers who vape believed vaping as less risky than cigarettes.
A 2017 report found that a minority of the respondents believed that
replacing cigarettes with e-cigarettes would be helpful for their
health. Many users vape because they believe it is healthier than smoking for themselves or bystanders. Usually, only a small proportion of users are concerned about the potential adverse health effects.
Some people say they want to quit smoking by vaping, but others vape to
circumvent smoke-free laws and policies, or to cut back on cigarette
smoking. 56% of respondents in a US 2013 survey had tried vaping to quit or reduce their smoking. In the same survey, 26% of respondents would use them in areas where smoking was banned.
Continuing dual use among smokers is correlated with trying to cut down
on smoking and to get around smoking bans, increased desire to quit
smoking, and a decreased smoking dependence. Seniors seem to vape to quit smoking or to get around smoke‐free policies. Concerns over avoiding stains on teeth or odor from smoke on clothes in some cases prompted interest in or use of e-cigarettes. Some e-cigarettes appeal considerably to people curious in technology who want to customize their devices.
There appears to be a hereditary component to tobacco use, which
probably plays a part in transitioning of e-cigarette use from
experimentation to routine use.
It is conceivable that former smokers may be tempted to use
nicotine again as a result of e-cigarettes, and possibly start smoking
again. E-liquid flavors are enticing to a range of smokers and non-smokers. Non-smoking adults tried e-cigarettes due to curiosity, because a relative was using them, or because they were given one. College students often vape for experimentation.
Millions of dollars spent on marketing aimed at smokers suggests
e-cigarettes are "newer, healthier, cheaper and easier to use in
smoke-free situations, all reasons that e-cigarette users claim motivate
their use".
Marketing messages echo well-established cigarette themes, including
freedom, good taste, romance, sexuality, and sociability as well as
messages stating that e-cigarettes are healthy, are useful for smoking
cessation, and can be used in smoke free environments. These messages are mirrored in the reasons that adults and youth cite for using e-cigarettes. Exposure to e-cigarette advertising influences people to try them.
Reasons for initiating e-cigarette use in the European Union according to a Eurobarometer poll (2018).
The belief that e-cigarettes are safer than traditional cigarettes could widen their use among pregnant women.
If tobacco businesses persuade women that e-cigarettes are a small
risk, non-smoking women of reproductive age might start using them and
women smoking during pregnancy might switch to their use or use these
devices to reduce smoking, instead of quitting smoking altogether.
Traditional cigarette users who have not used e-cigarettes had mixed
ideas about their possible satisfaction and around a third thought that
e-cigarettes might taste bad. Among current e-cigarette users, e-liquid flavor availability is very appealing. They feel or taste similar to traditional cigarettes, and vapers disagree about whether this is a benefit or a drawback. Some users like that e-cigarettes resembled traditional cigarettes, but others did not. E-cigarettes users' views about saving money from using e-cigarettes compared to traditional cigarettes are inconsistent.
The majority of committed e-cigarette users interviewed at an
e-cigarette convention found them cheaper than traditional cigarettes.
Some users stopped vaping due to issues with the devices. Dissatisfaction and concerns over safety can discourage ongoing e-cigarette use.
Commonly reported issues with using e-cigarettes were that the devices
were hard to refill, the cartridges might leak and that altering the
dose was hard.
Smokers mainly quit vaping because it did not feel similar to
traditional cigarettes, did not aid with cravings, and because they
wanted to use them only to know what they were like.
Progression
Many users may begin by using a disposable e-cigarette. Users often start with e-cigarettes resembling traditional cigarettes, eventually moving to a later-generation device. Most later-generation e-cigarette users shifted to their present device to get a "more satisfying hit", and users may adjust their devices to provide more vapor for better "throat hits". A 2014 study reported that experienced users preferred rechargeable e-cigarettes over disposable ones. The most commonly used e-cigarettes in the UK are devices with refillable tanks. Most users used either closed systems or open systems, and rarely used both. Women were found to prefer disposable e-cigarettes, and young adults were found to pay more attention to modifiability. Modifiability also was found to increase the probability of initiating e-cigarettes among adolescents.
A 2013 study found that about three-fourths of smokers used a
tank system, which allows users to choose flavors and strength to mix
their own liquid. Experienced e-cigarette users even ranked the ability to customize as the most important characteristic.
Users ranked nicotine strength as an important factor for choosing
among various e-cigarettes, though such preference could vary by smoking
status, e-cigarette use history, and gender.
Non-smokers and inexperienced e-cigarettes users tended to prefer no
nicotine or low nicotine e-cigarettes while smokers and experienced
e-cigarettes users preferred medium and high nicotine e-cigarettes. There is an abundance of colors, designs, carrying cases, and accessories to accommodate the diversity in personal preferences.
Construction
An e-cigarette is a handheld battery-powered vaporizer that simulates smoking, but without tobacco combustion. E-cigarette components include a mouthpiece, a cartridge (liquid storage area), a heating element/atomizer, a microprocessor, a battery, and some of them have an LED light on the end.
An exception to this are mechanical e-cigarettes (mods) which contain
no electronics and the circuit is closed by using a mechanical action
switch. An atomizer consists of a small heating element, or coil that vaporizes e-liquid and a wickiing material that draws liquid onto the coil. When the user inhales a flow sensor activates the heating element that atomizes the liquid solution; most devices are manually activated by a push-button. The e-liquid reaches a temperature of roughly 100-250 °C (212º-482 °F) within a chamber to create an aerosolized vapor. The user inhales an aerosol, which is commonly but inaccurately called vapor, rather than cigarette smoke.
Vaping is different than smoking, but there are some similarities,
including the hand-to-mouth action of smoking and a vapor that looks
like cigarette smoke. The aerosol provides a flavor and feel similar to smoking. A traditional cigarette is smooth and light but an e-cigarette is rigid, cold and slightly heavier. There is a learning curve to use e-cigarettes properly. E-cigarettes are cigarette-shaped, and there are many other variations. E-cigarettes that resemble pens or USB memory sticks are also sold that may be used unobtrusively.
There are multiple types of e-cigarettes: cigalikes that look
like cigarettes; tank models that are bigger than cigalikes and have
refillable liquid tanks; and mods, assembled from basic parts or by
altering existing products. Cigalikes are either disposable or come with rechargeable batteries and replaceable nicotine cartridges. A cigalike e-cigarette contains a cartomizer, which is connected to a battery. A "cartomizer" (a portmanteau of cartridge and atomizer) or "carto" consists of an atomizer surrounded by a liquid-soaked poly-foam that acts as an e-liquid holder. Clearomizers or "clearos", not unlike cartotanks, use a clear tank in which an atomizer is inserted.
A rebuildable atomizer or an RBA is an atomizer that allows the user to
assemble or "build" the wick and coil themselves instead of replacing
them with off-the-shelf atomizer "heads". The power source is the biggest component of an e-cigarette, which is frequently a rechargeable lithium-ion battery.
As the e-cigarette industry continues to evolve, new products are quickly developed and brought to market. First-generation e-cigarettes tend to look like traditional cigarettes and so are called "cigalikes". Most cigalikes look like cigarettes but there is some variation in size. Second-generation devices are larger overall and look less like traditional cigarettes. Third-generation devices include mechanical mods and variable voltage devices. The fourth-generation includes Sub ohm tanks (meaning that they have electrical resistance of less than 1 Ohm) and temperature control devices. The voltage for first-generation e-cigarettes is about 3.7 and second-generation e-cigarettes can be adjusted from 3 V to 6 V, while more recent devices can go up to 8 V. The latest generation of e-cigarettes are pod mods, which provide higher levels of nicotine than regular e-cigarettes through the production of aerosolized protonated nicotine.
E-liquid is the mixture used in vapor products such as e-cigarettes and usually contain propylene glycol, glycerin, nicotine, flavorings, additives, and differing amounts of contaminants. E-liquid formulations greatly vary due to fast growth and changes in manufacturing designs of e-cigarettes. The composition of the e-liquid for additives such as nicotine and flavors vary across and within brands.
The liquid typically consists of a combined total of 95% propylene
glycol and glycerin, and the remaining 5% being flavorings, nicotine,
and other additives. There are e-liquids sold without propylene glycol, nicotine, or flavors. The flavorings may be natural, artificial, or organic. Over 80 chemicals such as formaldehyde and metallic nanoparticles have been found in the e-liquid. There are many e-liquids manufacturers in the US and worldwide, and more than 15,500 flavors existed in 2018. Under the US Food and Drug Administration (US FDA) rules, e-liquid manufacturers are required to comply with a number of manufacturing standards. The revision to the EU Tobacco Products Directive has some standards for e-liquids. Industry standards have been created and published by the American E-liquid Manufacturing Standards Association (AEMSA).
Health effects
Positions of medical organizations
The scientific community in US and Europe are primarily concerned with their possible effect on public health. There is concern among public health experts that e-cigarettes could renormalize smoking, weaken measures to control tobacco, and serve as a gateway for smoking among youth. The public health community is divided over whether to support e-cigarettes, because their safety and efficacy for quitting smoking is unclear.
Many in the public health community acknowledge the potential for their
quitting smoking and decreasing harm benefits, but there remains a
concern over their long-term safety and potential for a new era of users
to get addicted to nicotine and then tobacco.
There is concern among tobacco control academics and advocates that
prevalent universal vaping "will bring its own distinct but as yet
unknown health risks in the same way tobacco smoking did, as a result of
chronic exposure", among other things.
Medical organizations differ in their views about the health implications of vaping. There is general agreement that e-cigarettes expose users to fewer toxicants than tobacco cigarettes.
Some healthcare groups and policy makers have hesitated to recommend
e-cigarettes for quitting smoking, because of limited evidence of
effectiveness and safety. Some have advocated bans on e-cigarette sales and others have suggested that e-cigarettes may be regulated as tobacco products but with less nicotine content or be regulated as a medicinal product. A 2016 World Health Organization (WHO) report found that the scientific evidence for the effectiveness of vaping for quitting smoking is "scant and of low certainty".
Healthcare organizations in the UK in 2015 have encouraged smokers to
try e-cigarettes to help them quit smoking and also encouraged
e-cigarette users to quit smoking tobacco entirely. In 2016, the US Food and Drug Administration
(US FDA) stated that "Although ENDS [electronic nicotine delivery
systems] may potentially provide cessation benefits to individual
smokers, no ENDS have been approved as effective cessation aids." In 2019 the European Respiratory Society
stated that "The long-term effects of ECIG use are unknown, and there
is therefore no evidence that ECIGs are safer than tobacco in the long
term." Following hundreds of possible cases of severe lung illness and five confirmed deaths associated with vaping in the US, the Centers for Disease Control and Prevention stated on September 6, 2019 that people should consider not using vaping products while their investigation is ongoing.
Smoking cessation
The available research on the safety and efficacy of e-cigarette use for smoking cessation is limited. Data regarding their use includes four randomized controlled trials and a number of user surveys, case reports, and cohort studies. There is tentative evidence they may help people quit smoking. Vaping does not greatly increase the odds of quitting smoking. Their use for quitting smoking is controversial. Studies determining whether e-cigarettes can help as a smoking cessation aid have come to conflicting conclusions. The evidence is conflicting to support or dismiss vaping for quit smoking.
As a result of the data being confronted with methodological and study
design limitations, no firm conclusions can be drawn in respect to their
efficacy and safety. A 2016 review found that the combined abstinence rate among smokers using e-cigarettes in prospective studies was 29.1%.
The same review noted that few clinical trials and prospective studies
had yet been conducted on their effectiveness, and only one randomized
clinical trial had included a group using other quit smoking methods. No long-term trials have been conducted for their use as a smoking cessation aid. It is still not evident as to whether vaping can adequately assist with quitting smoking at the population level.
A 2015 PHE report recommends for smokers who cannot or do not want to
quit to use e-cigarettes as one of the main steps to lower
smoking-related disease,
while a 2015 US PSTF statement found there is not enough evidence to
recommend e-cigarettes for quitting smoking in adults, pregnant women,
and adolescents. As of January 2018, systematic reviews
collectively agreed that there is insufficient evidence to
unequivocally determine whether vaping helped people abstain from
smoking.
Studies pertaining to their potential impact on smoking reduction are very limited. E-cigarette use may decrease the number of cigarettes smoked, but smoking just one to four cigarettes daily greatly increases the risk of cardiovascular disease compared to not smoking. The extent to which decreasing cigarette smoking with vaping leads to quitting is unknown. Randomized controlled trials have not shown that vaping is effective for quitting smoking. A 2016 meta-analysis
based on 20 different studies found that smokers who used e-cigarettes
were 28% less likely to quit than those who had not tried e-cigarettes. This finding persisted whether the smokers were initially interested in quitting or not.
It is unclear whether e-cigarettes are only helpful for particular types of smokers. Vaping with nicotine may reduce tobacco use among daily smokers.
Whether or not vaping is potentially effective for quitting smoking may
rely upon whether it was used as part of making an effort to quit or
not.
Vaping is not clearly more or less effective than regulated nicotine replacement products or 'usual care' for quitting smoking. The available research suggests e-cigarettes are likely equal or slightly better than nicotine patches for quitting smoking. People who vaped were not more likely to give up smoking than people who did not vape. Compared to many alternative quitting smoking medicines in early development in clinical trials including e-cigarettes, cytisine appears to be most encouraging in efficacy and safety with an inexpensive price. E-cigarettes have not been proven to be more effective than smoking cessation medicine and regulated US FDA medicine. A 2014 review found they may be as effective, but not more, compared to nicotine patches for short-term quitting smoking. They also found that a randomized trial stated 29% of e-cigarette users were still vaping at 6 months, while only 8% of patch users still wore patches at 6 months. Some individuals who quit smoking with a vaping device are continuing to vape after a year.
Vaping appears to be as effective as nicotine replacement products,
though its potential adverse effects such as normalizing smoking have
not been adequately studied.
While some surveys reported improved quitting smoking, particularly
with intensive e-cigarette users, several studies showed a decline in
quitting smoking in dual users.
A 2015 overview of systematic reviews indicates that e-cigarettes has
no benefit for smokers trying to quit, and that the high rate of dual
use indicates that e-cigarettes are used for supporting their nicotine
addiction. Other kinds of nicotine replacement products are usually covered by health systems, but because e-cigarettes are not medically licensed they are not covered.
It is difficult to reach a general conclusion from e-cigarette
use for smoking cessation because there are hundreds of brands and
models of e-cigarettes sold that vary in the composition of the liquid. E-cigarettes have not been subjected to the same type of efficacy testing as nicotine replacement products.
The similarity of e-cigarettes' vapor, looking like cigarette smoke,
may prolong traditional cigarette use for people who could have quit
instead, but the growing support of e-cigarettes could put extra
pressure on smokers to stop cigarette smoking because smoking may be
seen as socially unacceptable compared to a smokeless e-cigarette.
The evidence indicates smokers are more frequently able to completely
quit smoking using tank devices compared to cigalikes, which may be due
to their more efficient nicotine delivery. There is low quality evidence that vaping assists smokers to quit smoking in the long-term compared with nicotine-free vaping.
Nicotine-containing e-cigarettes were associated with greater
effectiveness for quitting smoking than e-cigarettes without nicotine.
A 2013 study in smokers who were not trying to quit, found that vaping,
with or without nicotine decreased the number of cigarettes consumed. E-cigarettes without nicotine may reduce tobacco cravings because of the smoking-related physical stimuli. A 2015 meta-analysis on clinical trials found that e-cigarettes containing nicotine are more effective than nicotine-free ones for quitting smoking.
They compared their finding that nicotine-containing e-cigarettes
helped 20% of people quit with the results from other studies that found
nicotine replacement products helps 10% of people quit. A 2016 review found low quality evidence of a trend towards benefit of e-cigarettes with nicotine for smoking cessation. In terms of whether flavored e-cigarettes assisted quitting smoking, the evidence is inconclusive.
The efficacy and safety of vaping for quitting smoking during pregnancy is unknown. No research is available to provide details on the efficacy of vaping for quitting smoking during pregnancy. There is robust evidence that vaping is not effective for quitting smoking among adolescents. In view of the shortage of evidence, vaping is not recommend for cancer patients. The effectiveness of vaping for quitting smoking among vulnerable groups is uncertain.
Harm reduction
Effects of vaping, compared to tobacco smoking.
The term harm reduction
implies any reduction in relative harm from a prior level, even a small
reduction such as reducing smoking by one or two cigarettes per day. Harm minimization strives to reduce harms to zero (i.e., ideally to no use and thus no harmful exposure).
When a consumer does not want to stop all nicotine use, then harm
minimization implies striving for the complete elimination of smoked
tobacco exposure by substituting it with the use of less harmful
noncombusted forms of nicotine instead of smoking. Tobacco harm reduction
(THR) may serve as a substitute for traditional cigarettes with lower
risk products to reduce tobacco-related death and disease. Tobacco harm reduction has been a controversial area of tobacco control. Health advocates have been slow to support a harm reduction method out of concern that tobacco companies cannot be trusted to sell products that will lower the risks associated with tobacco use. E-cigarettes can reduce smokers' exposure to carcinogens and other toxic chemicals found in tobacco. A large number of smokers want to reduce harm from smoking by using e-cigarettes. The argument for harm reduction does not take into account the adverse effects of nicotine. There cannot be a defensible reason for harm reduction in children who are vaping with a base of nicotine. Quitting smoking is the most effective strategy to tobacco harm reduction.
Tobacco smoke contains 100 known carcinogens, and 900 potentially
cancer causing chemicals, but e-cigarette vapor contains less of the
potential carcinogens than found in tobacco smoke.
A study in 2015 using a third-generation device found levels of
formaldehyde were greater than with cigarette smoke when adjusted to a
maximum power setting. E-cigarettes cannot be considered safe because there is no safe level for carcinogens. Due to their similarity to traditional cigarettes, e-cigarettes could play a valuable role in tobacco harm reduction.
However, the public health community remains divided concerning the
appropriateness of endorsing a device whose safety and efficacy for
smoking cessation remain unclear.
Overall, the available evidence supports the cautionary implementation
of harm reduction interventions aimed at promoting e-cigarettes as
attractive and competitive alternatives to cigarette smoking, while
taking measures to protect vulnerable groups and individuals.
The core concern is that smokers who could have quit entirely will develop an alternative nicotine addiction.
Dual use may be an increased risk to a smoker who continues to use even
a minimal amount of traditional cigarettes, rather than quitting. The promotion of vaping as a harm reduction aid is premature, while a 2011 review found they appear to have the potential to lower tobacco-related death and disease. Evidence to substantiate the potential of vaping to lower tobacco-related death and disease is unknown. The health benefits of reducing cigarette use while vaping is unclear. E-cigarettes could have an influential role in tobacco harm reduction.
The authors warned against the potential harm of excessive regulation
and advised health professionals to consider advising smokers who are
reluctant to quit by other methods to switch to e-cigarettes as a safer
alternative to smoking.
A 2014 review recommended that regulations for e-cigarettes could be
similar to those for dietary supplements or cosmetic products to not
limit their potential for harm reduction.
A 2012 review found e-cigarettes could considerably reduce traditional
cigarettes use and they likely could be used as a lower risk replacement
for traditional cigarettes, but there is not enough data on their
safety and efficacy to draw definite conclusions. There is no research available on vaping for reducing harm in high-risk groups such as people with mental disorders.
A 2014 PHE report concluded that hazards associated with products
currently on the market are probably low, and apparently much lower
than smoking. However, harms could be reduced further through reasonable product standards.
The British Medical Association encourages health professionals to
recommend conventional nicotine replacement therapies, but for patients
unwilling to use or continue using such methods, health professionals
may present e-cigarettes as a lower-risk option than tobacco smoking. The American Association of Public Health Physicians
(AAPHP) suggests those who are unwilling to quit tobacco smoking or
unable to quit with medical advice and pharmaceutical methods should
consider other nicotine containing products such as e-cigarettes and smokeless tobacco for long-term use instead of smoking.
A 2014 WHO report concluded that some smokers will switch completely to
e-cigarettes from traditional tobacco but a "sizeable" number will use
both.
This report found that such "dual use" of e-cigarettes and tobacco
"will have much smaller beneficial effects on overall survival compared
with quitting smoking completely."
Safety
Possible adverse effects of vaping.
The risks of e-cigarette use are uncertain. There is little data about their safety, and considerable variation among e-cigarettes and in their liquid ingredients and thus the contents of the aerosol delivered to the user. Reviews on the safety of e-cigarettes have reached significantly different conclusions. A 2014 WHO report cautioned about potential risks of using e-cigarettes. Regulated US FDA products such as nicotine inhalers may be safer than e-cigarettes, but e-cigarettes are generally seen as safer than combusted tobacco products such as cigarettes and cigars. The risk of early death is anticipated to be similar to that of smokeless tobacco. Since vapor does not contain tobacco and does not involve combustion, users may avoid several harmful constituents usually found in tobacco smoke, such as ash, tar, and carbon monoxide. However, e-cigarette use with or without nicotine cannot be considered harmless. Repeated exposure over a long time to e-cigarette vapor poses substantial potential risk.
The long-term effects of e-cigarette use are unknown. The risk from serious adverse events, including death, was reported in 2016 to be low. Serious adverse events related to e-cigarettes were hypotension, seizure,
chest pain, rapid heartbeat, disorientation, and congestive heart
failure but it was unclear the degree to which they were the result of
e-cigarettes. Less serious adverse effects include abdominal pain, headache, blurry vision, throat and mouth irritation, vomiting, nausea, and coughing. They may produce less adverse effects compared to tobacco products. E-cigarettes reduce lung function, but to a much lower extent than with traditional cigarettes, and they reduce cardiac muscle function and increase inflammation, but these changes were only substantial with traditional cigarettes. In 2019, an outbreak of severe lung illness across multiple states in the US has been linked to the use of vaping products. 39 deaths have been confirmed in this outbreak, as of November 5, 2019. A 2014 WHO report said, "ENDS [Electronic nicotine delivery system] use poses serious threats to adolescents and fetuses." Aside from toxicity exposure in normal use, there are also risks from misuse or accidents such as nicotine poisoning (especially among small children), contact with liquid nicotine, fires caused by vaporizer malfunction, and explosions resulting from extended charging, unsuitable chargers, or design flaws. Battery explosions are caused by an increase in internal battery temperature and some have resulted in severe skin burns. There is a small risk of battery explosion in devices modified to increase battery power.
Possible side effects of nicotine
The cytotoxicity of e-liquids varies, and contamination with various chemicals have been detected in the liquid. Metal parts of e-cigarettes in contact with the e-liquid can contaminate it with metal particles. Many chemicals including carbonyl compounds such as formaldehyde can inadvertently be produced when the nichrome wire (heating element) that touches the e-liquid is heated and chemically reacted with the liquid. Normal usage of e-cigarettes, and reduced voltage (3.0 V) devices generate very low levels of formaldehyde. The later-generation and "tank-style" e-cigarettes with a higher voltage (5.0 V) may generate equal or higher levels of formaldehyde compared to smoking. A 2015 PHE report found that high levels of formaldehyde only occurred in overheated "dry-puffing". Users detect the "dry puff" (also known as a "dry hit") and avoid it, and they concluded that "There is no indication that EC users are exposed to dangerous levels of aldehydes." However, e-cigarette users may "learn" to overcome the unpleasant taste due to elevated aldehyde formation, when the nicotine craving is high enough. E-cigarette users who use devices that contain nicotine are exposed to its potentially harmful effects. Nicotine is associated with cardiovascular disease, possible birth defects, and poisoning. In vitro studies of nicotine have associated it with cancer, but carcinogenicity has not been demonstrated in vivo. There is inadequate research to show that nicotine is associated with cancer in humans. The risk is probably low from the inhalation of propylene glycol and glycerin. No information is available on the long-term effects of the inhalation of flavors.
E-cigarettes create vapor that consists of fine and ultrafine particles of particulate matter, with the majority of particles in the ultrafine range. The vapor have been found to contain propylene glycol, glycerin, nicotine, flavors, small amounts of toxicants, carcinogens, and heavy metals, as well as metal nanoparticles, and other substances. Exactly what the vapor consists of varies in composition and concentration
across and within manufacturers, and depends on the contents of the
liquid, the physical and electrical design of the device, and user
behavior, among other factors. E-cigarette vapor potentially contains harmful chemicals not found in tobacco smoke. The majority of toxic chemicals found in cigarette smoke are absent in e-cigarette vapor. E-cigarette vapor contains lower concentrations of potentially toxic chemicals than with cigarette smoke. Those which are present, are mostly below 1% of the corresponding levels permissible by workplace safety standards. But workplace safety standards do not recognize exposure to certain vulnerable groups such as people with medical ailments, children, and infants who may be exposed to second-hand vapor.
Concern exists that some of the mainstream vapor exhaled by e-cigarette
users may be inhaled by bystanders, particularly indoors. E-cigarette use by a parent might lead to inadvertent health risks to offspring. A 2014 review recommended that e-cigarettes should be regulated for consumer safety. There is limited information available on the environmental issues around production, use, and disposal of e-cigarettes that use cartridges. E-cigarettes that are not reusable may contribute to the problem of electronic waste.
Graphic from the 2016 US Surgeon General's report entitled Aerosol and Other Risks.
Addiction and dependence
Nicotine is derived from the leaves of Nicotiana tabacum.
Nicotine, a key ingredient in most e-liquids, is well-recognized as one of the most addictive substances, as addictive as heroin and cocaine. Addiction is believed to be a disorder of experience-dependent brain plasticity. The reinforcing effects of nicotine play a significant role in the beginning and continuing use of the drug. First-time nicotine users develop a dependence about 32% of the time. Chronic nicotine use involves both psychological and physical dependence. Nicotine-containing e-cigarette vapor induces addiction-related neurochemical, physiological and behavioral changes. Nicotine affects neurological, neuromuscular, cardiovascular, respiratory, immunological and gastrointestinal systems. Neuroplasticity within the brain's reward system occurs as a result of long-term nicotine use, leading to nicotine dependence. The neurophysiological activities that are the basis of nicotine dependence are intricate. It includes genetic components, age, gender, and the environment.
Nicotine addiction is a disorder which alters different neural systems
such as dopaminergic, glutamatergic, GABAergic, serotoninergic, that
take part in reacting to nicotine.
Long-term nicotine use affects a broad range of genes associated with
neurotransmission, signal transduction, and synaptic architecture.
The ability to quitting smoking is affected by genetic factors,
including genetically based differences in the way nicotine is
metabolized.
The reinforcing effects of drugs of abuse, such as nicotine, are associated with its ability to excite the mesolimbic and dopaminergic systems.
How does the nicotine in e-cigarettes affect the brain? Until about age 25, the brain is still growing. Each time a new memory is created or a new skill is learned, stronger connections – or synapses – are built between brain cells. Young people's brains build synapses faster than adult brains. Because addiction is a form of learning, adolescents can get addicted more easily than adults. The nicotine in e-cigarettes and other tobacco products can also prime the adolescent brain for addiction to other drugs such as cocaine.
Nicotine is a parasympathomimetic stimulant that binds to and activates nicotinic acetylcholine receptors in the brain, which subsequently causes the release of dopamine and other neurotransmitters, such as norepinephrine, acetylcholine, serotonin, gamma-aminobutyric acid, glutamate, endorphins, and several neuropeptides, including proopiomelanocortin-derived α-MSH and adrenocorticotropic hormone. Corticotropin-releasing factor, Neuropeptide Y, orexins, and norepinephrine are involved in nicotine addiction.
Continuous exposure to nicotine can cause an increase in the number of
nicotinic receptors, which is believed to be a result of receptor desensitization and subsequent receptor upregulation. Long-term exposure to nicotine can also result in downregulation of glutamate transporter 1.
Long-term nicotine exposure upregulates cortical nicotinic receptors,
but it also lowers the activity of the nicotinic receptors in the
cortical vasodilation region. These effects are not easily understood. With constant use of nicotine, tolerance occurs at least partially as a result of the development of new nicotinic acetylcholine receptors in the brain. After several months of nicotine abstinence, the number of receptors go back to normal. The extent to which alterations in the brain caused by nicotine use are reversible is not fully understood. Nicotine also stimulates nicotinic acetylcholine receptors in the adrenal medulla, resulting in increased levels of epinephrine and beta-endorphin. Its physiological effects stem from the stimulation of nicotinic acetylcholine receptors, which are located throughout the central and peripheral nervous systems.
When nicotine intake stops, the upregulated nicotinic acetylcholine receptors induce withdrawal symptoms.
These symptoms can include cravings for nicotine, anger, irritability,
anxiety, depression, impatience, trouble sleeping, restlessness, hunger,
weight gain, and difficulty concentrating.
When trying to quit smoking with vaping a base containing nicotine,
symptoms of withdrawal can include irritability, restlessness, poor
concentration, anxiety, depression, and hunger. The changes in the brain cause a nicotine user to feel abnormal when not using nicotine. In order to feel normal, the user has to keep his or her body supplied with nicotine. E-cigarettes may reduce cigarette craving and withdrawal symptoms. It is not clear whether e-cigarette use will decrease or increase overall nicotine addiction, but the nicotine content in e-cigarettes is adequate to sustain nicotine dependence. Chronic nicotine use causes a broad range of neuroplastic adaptations, making quitting hard to accomplish. A 2015 study found that users vaping non-nicotine e-liquid exhibited signs of dependence. Experienced users tend to take longer puffs which may result in higher nicotine intake.
It is difficult to assess the impact of nicotine dependence from
e-cigarette use because of the wide range of e-cigarette products. The addiction potential of e-cigarettes may have risen because as they have progressed, they delivery nicotine better.
A 2015 American Academy of Pediatrics (AAP) policy statement
stressed "the potential for these products to addict a new generation
of youth to nicotine and reverse more than 50 years of public health
gains in tobacco control." The World Health Organization (WHO) is concerned about starting nicotine use among non-smokers, and the National Institute on Drug Abuse said e-cigarettes could maintain nicotine addiction in those who are attempting to quit. The limited available data suggests that the likelihood of abuse from e-cigarettes is smaller than traditional cigarettes. No long-term studies have been done on the effectiveness of e-cigarettes in treating tobacco addiction,
but some evidence suggests that dual use of e-cigarettes and
traditional cigarettes may be associated with greater nicotine
dependence.
Following the possibility of nicotine addiction via e-cigarettes, there is concern that children may start smoking cigarettes. Adolescents are likely to underestimate nicotine's addictiveness.
Vulnerability to the brain-modifying effects of nicotine, along with
youthful experimentation with e-cigarettes, could lead to a lifelong
addiction. A long-term nicotine addiction from using a vape may result in using other tobacco products. The majority of addiction to nicotine starts during youth and young adulthood. Adolescents are more likely to become nicotine dependent than adults. The adolescent brain seems to be particularly sensitive to neuroplasticity as a result of nicotine. Minimal exposure could be enough to produce neuroplastic alterations in the very sensitive adolescent brain. A 2014 review found that in studies up to a third of youth who have not tried a traditional cigarette have used e-cigarettes.
The degree to which teens are using e-cigarettes in ways the
manufacturers did not intend, such as increasing the nicotine delivery,
is unknown, as is the extent to which e-cigarette use may lead to addiction or substance dependence in youth.
History
In 1927, Joseph Robinson applied for a patent for an electronic vaporizer. Its purpose was to be used with medicinal compounds. The patent was approved in 1930. The device was never made available for sale. In 1930, the United States Patent and Trademark Office
reported a patent stating, "for holding medicinal compounds which are
electrically or otherwise heated to produce vapors for inhalation."
In 1934, a patent stated that a product was "adapted for transforming
volatile liquid medicaments into vapors or into mists of exceedingly
fine particles." In 1936, a comparable patent was reported. These instances are in regard to vaporization for medicinal use. The earliest e-cigarette can be traced to American Herbert A. Gilbert,
who in 1963 patented "a smokeless non-tobacco cigarette" that involved
"replacing burning tobacco and paper with heated, moist, flavored air". This device produced flavored steam without nicotine. The patent was granted in 1965. Gilbert's invention was ahead of its time. There were prototypes, but it received little attention and was never commercialized because smoking was still fashionable at that time. Gilbert said in 2013 that today's electric cigarettes follow the basic design set forth in his original patent.
The Favor cigarette, introduced in 1986, was another early
noncombustible product promoted as an alternative nicotine-containing
tobacco product.
Hon Lik, a Chinese pharmacist and inventor, who worked as a research pharmacist for a company producing ginseng products, is frequently credited with the invention of the modern e-cigarette. But tobacco companies have been developing nicotine aerosol generation devices since as early as 1963.
Philip Morris' division NuMark, launched in 2013 the MarkTen
e-cigarette that Philip Morris had been working on since 1990, 13 years
prior to Hon Lik creating his e-cigarette. Hon quit smoking after his father, also a heavy smoker, died of lung cancer. In 2001, he thought of using a high frequency, piezoelectric ultrasound-emitting element to vaporize a pressurized jet of liquid containing nicotine. This design creates a smoke-like vapor.
Hon said that using resistance heating obtained better results and the
difficulty was to scale down the device to a small enough size. Hon's invention was intended to be an alternative to smoking. Hon Lik sees the e-cigarette as comparable to the "digital camera taking over from the analogue camera."
The Ruyan e-cigar was first launched in China in 2004.
Hon Lik registered a patent for the modern e-cigarette design in 2003. Hon is credited with developing the first commercially successful electronic cigarette. The e-cigarette was first introduced to the Chinese domestic market in 2004. Many versions made their way to the US, sold mostly over the Internet by small marketing firms. E-cigarettes entered the European market and the US market in 2006 and 2007. The company that Hon worked for, Golden Dragon Holdings, registered an international patent in November 2007. The company changed its name to Ruyan (如烟, literally "like smoke") later the same month, and started exporting its products.
Many US and Chinese e-cigarette makers copied his designs illegally, so
Hon has not received much financial reward for his invention (although
some US manufacturers have compensated him through out of court
settlements). Ruyan later changed its company name to Dragonite International Limited. Most e-cigarettes today use a battery-powered heating element rather than the earlier ultrasonic technology design.
Initially, their performance did not meet the expectations of users. The e-cigarette continued to evolve from the first-generation three-part device. In 2007 British entrepreneurs Umer and Tariq Sheikh invented the cartomizer. This is a mechanism that integrates the heating coil into the liquid chamber. They launched this new device in the UK in 2008 under their Gamucci brand and the design is now widely adopted by most "cigalike" brands. Other users tinkered with various parts to produce more satisfactory homemade devices, and the hobby of "modding" was born.
The first mod to replace the e-cigarette's case to accommodate a
longer-lasting battery, dubbed the "screwdriver", was developed by Ted
and Matt Rogers in 2008. Other enthusiasts built their own mods to improve functionality or aesthetics. When pictures of mods appeared at online vaping forums many people wanted them, so some mod makers produced more for sale.
In 2008, a consumer created an e-cigarette called the screwdriver. The device generated a lot of interest back then, as it let the user to vape for hours at one time.
The invention led to demand for customizable e-cigarettes, prompting
manufacturers to produce devices with interchangeable components that
could be selected by the user. In 2009, Joyetech developed the eGo series which offered the power of the screwdriver model and a user-activated switch to a wide market. The clearomizer was invented in 2009.
Originating from the cartomizer design, it contained the wicking
material, an e-liquid chamber, and an atomizer coil within a single
clear component. The clearomizer allows the user to monitor the liquid level in the device. Soon after the clearomizer reached the market, replaceable atomizer coils and variable voltage batteries were introduced. Clearomizers and eGo batteries became the best-selling customizable e-cigarette components in early 2012.
International tobacco companies dismissed e-cigarettes as a fad at first. However, recognizing the development of a potential new market sector that could render traditional tobacco products obsolete, they began to produce and market their own brands of e-cigarettes and acquire existing e-cigarette companies. They bought the largest e-cigarette companies. blu eCigs, a prominent US e-cigarette manufacturer, was acquired by Lorillard Inc. for $135 million in April 2012. British American Tobacco was the first tobacco business to sell e-cigarettes in the UK. They launched the e-cigarette Vype in July 2013. They launched Vype in 2013, while Imperial Tobacco's
Fontem Ventures acquired the intellectual property owned by Hon Lik
through Dragonite International Limited for $US 75 million in 2013 and
launched Puritane in partnership with Boots UK. On October 1, 2013 Lorillard Inc. acquired another e-cigarette company, this time the UK based company SKYCIG. SKY was rebranded as blu. On February 3, 2014, Altria Group, Inc. acquired popular e-cigarette brand Green Smoke for $110 million. The deal was finalized in April 2014 for $110 million with $20 million in incentive payments. Altria also markets its own e-cigarette, the MarkTen, while Reynolds American has entered the sector with its Vuse product. Philip Morris, the world's largest tobacco company, purchased UK's Nicocigs in June 2014. On April 30, 2015, Japan Tobacco bought the US Logic e-cigarette brand. Japan Tobacco also bought the UK E-Lites brand in June 2014. On July 15, 2014, Lorillard sold blu to Imperial Tobacco as part of a deal for $7.1 billion.
Society and culture
Consumers of e-cigarettes have shown passionate support for
e-cigarettes that other nicotine replacement products did not receive. They have the potential mass appeal that could challenge combustible tobacco's market position.
A subculture has emerged which calls itself "the vaping community". Members of this emerging subculture often see e-cigarettes as a safer alternative to smoking, and some view it as a hobby. The online forum E-Cig-Reviews.com was one of the first major communities. It and other online forums, such as UKVaper.org, were the origins of the hobby of modding. There are also groups on Facebook and Reddit. Online forums based around modding have grown in the vaping community.
Vapers energetically embrace activities associated with e-cigarettes
and in some circumstances act as unpaid evangelicals, according to a
2011 study.
E-cigarette companies have a substantial online presence, as well as
many individual vapers who blog and tweet about e-cigarette related
products. A 2014 Postgraduate Medical Journal
editorial stated that vapers "also engage in grossly offensive online
attacks on anyone who has the temerity to suggest that ENDS are anything
other than an innovation that can save thousands of lives with no
risks". Contempt for Big Tobacco is part of vaping culture. A 2014 review stated that tobacco and e-cigarette companies interact with consumers for their policy agenda.
The companies use websites, social media, and marketing to get
consumers involved in opposing bills that include e-cigarettes in
smoke-free laws. This is similar to tobacco industry activity going back to the 1980s. These approaches were used in Europe to minimize the EU Tobacco Products Directive in October 2013. Grassroots lobbying also influenced the Tobacco Products Directive decision.
Tobacco companies have worked with organizations conceived to promote
e-cigarette use, and these organizations have worked to hamper
legislation intended at restricting e-cigarette use.
A popular vaporizer used by American youth is the Juul. Close to 80% of respondents in a 2017 Truth Initiative study aged 15–24 reported using Juul also used the device in the last 30 days. Teenagers use the verb "Juuling" to describe their use of Juul, and Juuling is the subject of many memes on social media. Students commented on Twitter about using the Juul device in class.
E-cigarette user blowing a cloud of aerosol (vapor). The activity is known as cloud-chasing.
Large gatherings of vapers, called vape meets, take place around the US. They focus on e-cigarette devices, accessories, and the lifestyle that accompanies them. Vapefest, which started in 2010, is an annual show hosted by different cities. People attending these meetings are usually enthusiasts that use specialized, community-made products not found in convenience stores or gas stations.
These products are mostly available online or in dedicated "vape"
storefronts where mainstream e-cigarettes brands from the tobacco
industry and larger e-cig manufacturers are not as popular. Some vape shops have a vape bar where patrons can test out different e-liquids and socialize.
The Electronic Cigarette Convention in North America which started in
2013, is an annual show where companies and consumers meet up.
A subclass of vapers configure their atomizers to produce large amounts of vapor by using low-resistance heating coils. This practice is called "cloud-chasing". By using a coil with very low resistance, the batteries are stressed to a potentially unsafe extent. This could present a risk of dangerous battery failures.
As vaping comes under increased scrutiny, some members of the vaping
community have voiced their concerns about cloud-chasing, stating the
practice gives vapers a bad reputation when doing it in public. The Oxford Dictionaries' word of the year for 2014 was "vape".
Regulation
A no smoking or vaping sign from the US.
Regulation of e-cigarettes varies across countries and states, ranging from no regulation to banning them entirely. For instance, e-cigarettes are illegal in Japan, forcing the market to use heat-not-burn tobacco products for cigarette alternatives. Others have introduced strict restrictions and some have licensed devices as medicines such as in the UK. However, as of February 2018,
there is no e-cigarette device that has been given a medical license
that is commercially sold or available by prescription in the UK. As of 2015, around two thirds of major nations have regulated e-cigarettes in some way.
Because of the potential relationship with tobacco laws and medical
drug policies, e-cigarette legislation is being debated in many
countries. The companies that make e-cigarettes have been pushing for laws that support their interests. In 2016 the US Department of Transportation banned the use of e-cigarettes on commercial flights. This regulation applies to all flights to and from the US.
In 2018, the Royal College of Physicians asked that a balance is found
in regulations over e-cigarettes that ensure product safety while
encouraging smokers to use them instead of tobacco, as well as keep an
eye on any effects contrary to the control agencies for tobacco.
The legal status of e-cigarettes is currently pending in many countries. Many countries such as Brazil, Singapore, the Seychelles, Uruguay, and India have banned e-cigarettes. Canada-wide in 2014, they were technically illegal to sell, as no nicotine-containing e-cigarettes are not regulated by Health Canada, but this is generally unenforced and they are commonly available for sale Canada-wide. In 2016, Health Canada announced plans to regulate vaping products. In the US and the UK, the use and sale to adults of e-cigarettes are legal. The revised EU Tobacco Products Directive came into effect in May 2016, providing stricter regulations for e-cigarettes.
It limits e-cigarette advertising in print, on television and radio,
along with reducing the level of nicotine in liquids and reducing the
flavors used. It does not ban vaping in public places.
It requires the purchaser for e-cigarettes to be at least 18 and does
not permit buying them for anyone less than 18 years of age. The updated Tobacco Products Directive has been disputed by tobacco lobbyists whose businesses could be impacted by these revisions. As of August 8, 2016, the US FDA extended its regulatory power to include e-cigarettes, e-liquid and all related products.
Under this ruling the FDA will evaluate certain issues, including
ingredients, product features and health risks, as well their appeal to
minors and non-users. The FDA rule also bans access to minors. A photo ID is now required to buy e-cigarettes, and their sale in all-ages vending machines is not permitted in the US.
As of August 2017, regulatory compliance deadlines relating to
premarket review requirements for most e-cigarette and e-liquid products
have been extended from November 2017 to August 8, 2022, which attracted a lawsuit filed by the American Heart Association, American Academy of Pediatrics, the Campaign for Tobacco-Free Kids, and other plaintiffs. In May 2016 the US FDA used its authority under the Family Smoking Prevention and Tobacco Control Act
to deem e-cigarette devices and e-liquids to be tobacco products, which
meant it intended to regulate the marketing, labelling, and manufacture
of devices and liquids; vape shops that mix e-liquids or make or modify
devices were considered manufacturing sites that needed to register
with US FDA and comply with good manufacturing practice regulation.
E-cigarette and tobacco companies have recruited lobbyists in an effort
to prevent the US FDA from evaluating e-cigarette products or banning
existing products already on the market.
In February 2014 the European Parliament
passed regulations requiring standardization and quality control for
liquids and vaporizers, disclosure of ingredients in liquids, and
child-proofing and tamper-proofing for liquid packaging. In April 2014 the US FDA published proposed regulations for e-cigarettes. In the US some states tax e-cigarettes as tobacco products, and some state and regional governments have broadened their indoor smoking bans to include e-cigarettes. As of April 2017,
12 US states and 615 localities had prohibited the use of e-cigarettes
in venues in which traditional cigarette smoking was prohibited. In 2015, at least 48 states and 2 territories had banned e-cigarette sales to minors.
E-cigarettes containing nicotine have been listed as drug
delivery devices in a number of countries, and the marketing of such
products has been restricted or put on hold until safety and efficacy
clinical trials are conclusive. Since they do not contain tobacco, television advertising in the US is not restricted. Some countries have regulated e-cigarettes as a medical product even though they have not approved them as a smoking cessation aid.
A 2014 review stated the emerging phenomenon of e-cigarettes has raised
concerns in the health community, governments, and the general public
and recommended that e-cigarettes should be regulated to protect
consumers.
It added, "heavy regulation by restricting access to e-cigarettes would
just encourage continuing use of much unhealthier tobacco smoking." A 2014 review said regulation of the e-cigarette should be considered on the basis of reported adverse health effects.
Marketing
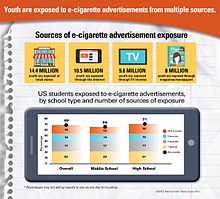
Adolescents are exposed to e-cigarette marketing in a number of ways, many of which are not available to traditional tobacco.
They are marketed to men, women, and children as being safer than traditional cigarettes. They are also marketed to non-smokers. E-cigarette marketing is common. There are growing concerns that e-cigarette advertising campaigns unjustifiably focus on young adults, adolescents, and women. Large tobacco companies have greatly increased their marketing efforts. This marketing trend may expand the use of e-cigarettes and contribute to re-glamorizing smoking. Some companies may use e-cigarette advertising to advocate smoking, deliberately, or inadvertently, is an area of concern.
A 2014 review said, "the e-cigarette companies have been rapidly
expanding using aggressive marketing messages similar to those used to promote cigarettes in the 1950s and 1960s."
E-cigarette companies are using methods that were once used by the
tobacco industry to persuade young people to starting using cigarettes. E-cigarettes are promoted to a certain extent to forge a vaping culture that entices non-smokers.
Themes in e-cigarette marketing, including sexual content and customer
satisfaction, are parallel to themes and techniques that have been found
to be appealing to youth and young adults in traditional cigarette
advertising and promotion.
A 2017 review found "The tobacco industry sees a future where ENDS
accompany and perpetuate, rather than supplant, tobacco use, especially
targeting the youth."
E-cigarettes and nicotine are regularly promoted as safe and even
healthy in the media and on brand websites, is an area of concern.
While advertising of tobacco
products is banned in most countries, television and radio e-cigarette
advertising in several countries may be indirectly encouraging
traditional cigarette use. E-cigarette advertisements are also in magazines, newspapers, online, and in retail stores. Between 2010 and 2014, e-cigarettes were second only to cigarettes as the top advertised product in magazines.
As cigarette companies have acquired the largest e-cigarette brands,
they currently benefit from a dual market of smokers and e-cigarette
users while simultaneously presenting themselves as agents of harm
reduction. This raises concerns about the appropriateness of endorsing a product that directly profits the tobacco industry.
There is no evidence that the cigarette brands are selling e-cigarettes
as part of a plan to phase out traditional cigarettes, despite some
stating to want to cooperate in "harm reduction".
E-cigarette advertising for using e-cigarettes as a quitting tool have
been seen in the US, UK, and China, which have not been supported by
regulatory bodies. In the US, six large e-cigarette businesses spent $59.3 million on promoting e-cigarettes in 2013. In the US and Canada, over $2 million is spent yearly on promoting e-cigarettes online. E-cigarette websites often made unscientific health statements in 2012. The ease to get past the age verification system at e-cigarette company websites allows underage individuals to access and be exposed to marketing. Around half of e-cigarette company websites have a minimum age notice that prohibited underage individuals from entering.
Celebrity endorsements are used to encourage e-cigarette use. A 2012 national US television advertising campaign for e-cigarettes starred Stephen Dorff
exhaling a "thick flume" of what the advertisement describes as "vapor,
not tobacco smoke", exhorting smokers with the message "We are all
adults here, it's time to take our freedom back."
Opponents of the tobacco industry state that the blu advertisement, in a
context of longstanding prohibition of tobacco advertising on
television, seems to have resorted to advertising tactics that got
former generations of people in the US addicted to traditional
cigarettes.
Cynthia Hallett of Americans for Non-Smokers' Rights described the US
advertising campaign as attempting to "re-establish a norm that smoking
is okay, that smoking is glamorous and acceptable".
University of Pennsylvania communications professor Joseph Cappella
stated that the setting of the advertisement near an ocean was meant to
suggest an association of clean air with the nicotine product. In 2012 and 2013, e-cigarette companies advertised to a large television audience in the US which included 24 million youth.
The channels to which e-cigarette advertising reached the largest
numbers of youth (ages 12–17) were AMC, Country Music Television, Comedy
Central, WGN America, TV Land, and VH1.
E-cigarette use among youth is rising as e-cigarette advertising increases.
E-cigarettes are heavily promoted across all media outlets.
They are vigorously advertised, mostly through the Internet, as a safe
substitute to traditional cigarettes, among other things. E-cigarette companies promote their e-cigarette products on Facebook, Instagram, YouTube, and Twitter. They are promoted on YouTube by movies with sexual material and music icons, who encourage minors to "take their freedom back." They have partenered with a number of sports and music icons to promote their products. Tobacco companies intensely market e-cigarettes to youth, with industry strategies including cartoon characters and candy flavors. Fruit flavored e-liquid is the most commonly marketed e-liquid flavor on social media.
E-cigarette companies commonly promote that their products contain only
water, nicotine, glycerin, propylene glycol, and flavoring but this
assertion is misleading as researchers have found differing amounts of
heavy metals in the vapor, including chromium, nickel, tin, silver,
cadmium, mercury, and aluminum.
The widespread assertion that e-cigarettes emit "only water vapor" is
not true because the evidence demonstrates e-cigarette vapor contains
possibly harmful chemicals such as nicotine, carbonyls, metals, and volatile organic compounds, in addition to particulate matter. Massive advertising included the assertion that they would present little risk to non-users.
Though, "disadvantages and side effects have been reported in many
articles, and the unfavorable effects of its secondhand vapor have been
demonstrated in many studies." Many e-cigarette companies market their products as a smoking cessation aid without evidence of effectiveness.
E-cigarette marketing has been found to make unsubstantiated health
statements (e.g., that they help one quit smoking) including statements
about improving psychiatric symptoms, which may be particularly
appealing to smokers with mental illness. E-cigarette marketing advocate weight control and emphasize use of nicotine with many flavors. These marketing angles could particularly entice overweight people, youth, and vulnerable groups. Some e-cigarette companies state that their products are green without supporting evidence which may be purely to increase their sales.
Economics
Vaping stand in London shopping centre.
The number of e-cigarettes sold increased every year from 2003 to 2014. In 2015 a slowdown in the growth in usage occurred in the US. As of January 2018, the growth in usage in the UK has slowed down since 2013. As of 2014 there were at least 466 e-cigarette brands. Worldwide e-cigarette sales in 2014 were around US$7 billion. E-cigarette sales could exceed traditional cigarette sales by 2023. Approximately 30–50% of total e-cigarettes sales are handled on the internet. Established tobacco companies have a significant share of the e-cigarette market.
As of 2018, 95% of e-cigarette devices were made in China, mainly in Shenzhen. Chinese companies' market share of e-liquid is low. In 2014, online and offline sales starting increases.
Since combustible cigarettes are relatively inexpensive in China a
lower price may not be large factor in marketing vaping products over
there.
In 2015, 80% of all e-cigarette sales in convenience stores in the US were products made by tobacco companies. According to Nielsen Holdings, convenience store e-cigarette sales in the US went down for the first time during the four-week period ending on 10 May 2014.
Wells Fargo analyst Bonnie Herzog attributes this decline to a shift in
consumers' behavior, buying more specialized devices or what she calls
"vapors-tanks-mods (VTMs)" that are not tracked by Nielsen. Wells Fargo estimated that VTMs accounted for 57% of the 3.5 billion dollar market in the US for vapor products in 2015.
In 2014, dollar sales of customizable e-cigarettes and e-liquid
surpassed sales of cigalikes in the US, even though, overall,
customizables are a less expensive vaping option.
In 2014, the Smoke-Free Alternatives Trade Association estimated that
there were 35,000 vape shops in the US, more than triple the number a
year earlier. However the 2015 slowdown in market growth affected VTMs as well. Large tobacco retailers are leading the cigalike market.
"We saw the market's sudden recognition that the cigarette industry
seems to be in serious trouble, disrupted by the rise of vaping," Mad Money's Jim Cramer stated April 2018.
"Over the course of three short days, the tobacco stocks were bent,
they were spindled and they were mutilated by the realization that
electronic cigarettes have become a serious threat to the old-school
cigarette makers," he added. The leading seller in the e-cigarette market in the US is the Juul e-cigarette, which was introduced in June 2015. As of August 2018, Juul accounts for over 72% of the US e-cigarette market monitored by Nielsen, and its closest competitor—RJ Reynolds' Vuse—makes up less than 10% of the market. Juul rose to popularity quickly, growing by 700% in 2016 alone. On July 17, 2018 Reynolds announced it will debut in August 2018 a pod mod type device similar Juul. The popularity of the Juul pod system has led to a flood of other pod devices hitting the market.
In Canada, e-cigarettes had an estimated value of 140 million CAD in 2015. There are numerous e-cigarette retail shops in Canada.
A 2014 audit of retailers in four Canadian cities found that 94% of
grocery stores, convenience stores, and tobacconist shops which sold
e-cigarettes sold nicotine-free varieties only, while all vape shops
stocked at least one nicotine-containing product.
By 2015 the e-cigarette market had only reached a twentieth of the size of the tobacco market in the UK.
In the UK in 2015 the "most prominent brands of cigalikes" were owned
by tobacco companies, however, with the exception of one model, all the
tank types came from "non-tobacco industry companies". Yet some tobacco industry products, while using prefilled cartridges, resemble tank models.
France's e-cigarette market was estimated by Groupe Xerfi to be €130 million in 2015. Additionally, France's e-liquid market was estimated at €265 million. In December 2015, there were 2,400 vape shops in France, 400 fewer than in March of the same year. Industry organization Fivape said the reduction was due to consolidation, not to reduced demand.
Related technologies
An e-hookah user.
Philip Morris International's IQOS device with charger and tobacco stick.
Other devices to deliver inhaled nicotine have been developed. They aim to mimic the ritual and behavioral aspects of traditional cigarettes.
British American Tobacco, through their subsidiary Nicoventures, licensed a nicotine delivery system based on existing asthma inhaler technology from UK-based healthcare company Kind Consumer.
In September 2014 a product based on this named Voke obtained approval
from the United Kingdom's Medicines and Healthcare Products Regulatory
Agency.
In 2011 Philip Morris International bought the rights to a nicotine pyruvate technology developed by Jed Rose at Duke University. The technology is based on the chemical reaction between pyruvic acid and nicotine, which produces an inhalable nicotine pyruvate vapor. Philip Morris Products S.A. created a different kind e-cigarette named P3L. The device is supplied with a cartridge that contains nicotine and lactic acid in different cavities. When turned on and heated, the nicotine salt called nicotine lactate forms an aerosol.
The IQOS is a heat-not-burn tobacco product marketed by Philip Morris International. It heats tobacco at a lower temperature than traditional cigarettes. The tobacco sticks reach a temperature up to 350 °C. It sold first in Japan since November 2014.
On December 2, 2016, the United Tobacco Vapor Group's (UTVG) stated
that they have been given a patent for their vaporizing component
system. qmos from UTVG does not contain a wick or sponge and the number of components is 5 compared to 20 for traditional e-cigarettes. Pax Labs has developed vaporizers that heats the leaves of tobacco to deliver nicotine in a vapor. On June 1, 2015, they introduced Juul a type of e-cigarette which delivers 10 times as much nicotine as other e-cigarettes, equivalent to an actual cigarette puff. Juul was spun off from Pax Labs in June 2017 and is now available by the independent company Juul Labs. The eTron 3T from Vapor Tobacco Manufacturing, launched in December 2014, employs a patented, aqueous system whereby the tobacco is extracted into water. The e-liquid contains organic tobacco, organic glycerin, and water.
In December 2013 Japan Tobacco launched Ploom in Japan. In January 2016 they launched Ploom TECH that produces a vapor from a heated liquid that moves through a capsule of granulated tobacco leaves. In 2016 British American Tobacco (BAT) released its own version of the heat but not burn technology called glo in Japan and Switzerland. It uses tobacco sticks rather than nicotine liquid, and does not directly heat or burn tobacco. In 2015 the glo iFuse was released in Romania by BAT. It uses a coil to heat a liquid that produces a vapor that moves through mixed tobacco. Heat-not-burn tobacco products were first introduced in 1988, but were not a commercial success.
BLOW started selling e-hookahs, an electronic version of the hookah, in 2014. The handle of each hose for the e-hookah contains a heating element and a liquid, which produces vapor. Gopal Bhatnagar based in Toronto, Canada invented a 3D printed adapter to turn a traditional hookah into an e-hookah. It is used instead of the ceramic bowl that contains shisha tobacco. Rather than the tobacco, users can insert e-cigarettes. KanaVape is an e-cigarette containing cannabidiol (CBD) and no THC. Several companies including Canada's Eagle Energy Vapor are selling caffeine-based e-cigarettes instead of containing nicotine.