In microeconomics, diseconomies of scale are the cost disadvantages that economic actors accrue due to an increase in organizational size or in output, resulting in production of goods and services at increased per-unit costs. The concept of diseconomies of scale is the opposite of economies of scale. In business, diseconomies of scale are the features that lead to an increase in average costs as a business grows beyond a certain size.
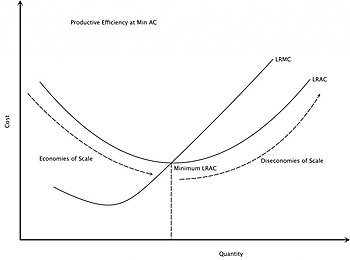
The rising part of the long-run average cost
curve illustrates the effect of diseconomies of scale. The Long Run
Average Cost (LRAC) curve plots the average cost of producing the lowest
cost method. The Long Run Marginal Cost (LRMC) is the change in total
cost attributable to a change in the output of one unit after the plant
size has been adjusted to produce that rate of output at minimum LRAC.
Causes
Communication costs
Ideally,
all employees of a firm would have one-on-one communication with each
other so they know exactly what the other workers are doing. A firm with
a single worker does not require any communication between employees. A
firm with two workers requires one communication channel, directly
between those two workers. A firm with three workers requires three
communication channels between employees (between employees A & B, B
& C, and A & C). Here is a chart of one-on-one communication
channels required:
| Workers | Communication Channels |
|---|---|
| 1 | 0 |
| 2 | 1 |
| 3 | 3 |
| 4 | 6 |
| 5 | 10 |
| n |
The graph of all one-on-one channels is a complete graph.
The number of one-on-one channels of communication grows more
rapidly than the number of workers, thus increasing the time and costs
of communication. At some point one-on-one communications between all
workers becomes impractical; therefore only certain groups of employees
will communicate with one another (e.g. within departments or within
geographical locations). This reduces, but does not stop, the increase
in unit costs; and also the organisation will incur some inefficiencies
due to the reduced level of communication.
Duplication of effort
An organisation with just one person cannot have any duplication of effort
between employees. If there are two employees, there could be some
duplication of efforts, but this is likely to be minor, as each of the
two will generally know what the other is working on. When organisations
grow to thousands of workers, it is inevitable that someone, or even a
team, will take on a function that is already being handled by another
person or team. In colloquial terms, this is described as "one hand not
knowing what the other hand is doing". General Motors, for example, developed two in-house CAD/CAM
systems: CADANCE was designed by the GM Design Staff, while Fisher
Graphics was created by the former Fisher Body division. These similar
systems later needed to be combined into a single Corporate Graphics
System, CGS, at great expense. A smaller firm would have had neither the
money to allow such expensive parallel developments, nor the lack of
communication and cooperation which precipitated this event. In addition
to CGS, GM also used CADAM, UNIGRAPHICS, CATIA
and other off-the-shelf CAD/CAM systems, thus increasing the cost of
translating designs from one system to another. This endeavor eventually
became so unmanageable that they acquired (and then eventually sold
off) Electronic Data Systems
(EDS) in an effort to control the situation. Smaller firms typically
choose a single off-the-shelf CAD/CAM system, with no need to combine or
translate between systems.
Office politics
"Office politics"
is management behavior which a manager knows is counter to the best
interest of the company, but is in his personal best interest. For
example, a manager might intentionally promote an incompetent worker,
knowing that the worker will never be able to compete for the manager's
job. This type of behavior only makes sense in a company with multiple
levels of management. The more levels there are, the more opportunity
for this behavior. In a small company, such behavior could cause the
company to go bankrupt, and thus cost the manager his job, so he would
not make such a decision. In a large company, one manager would not have
much effect on the overall health of the company, so such "office
politics" are in the interest of individual managers.
Top-heavy companies
As
an organisation increases in size, it becomes costly to keep control of
a sprawling corporate empire, and this often results in bureaucracy as
executives implement more and more levels of management. As firms
increase in size, managers will initially provide a net benefit to the
firm and increase productivity; however, as a firm grows and covers a
larger geographical area and/or employs more people, a principal–agent problem
arises, leading to lower productivity. To counter this, executives
introduce standards and controls in order to maintain productivity, and
this necessitates the hiring of more managers to apply these standards
and controls, hence the proportion of managerial to working class begins
to lean towards managerial and the company becomes "top-heavy".
However, these additional managers are not providing additional output:
they are spending their time implementing standards and carrying out
supervision that is unnecessary in smaller firms, hence the
cost-per-unit has increased.
Supply-chain disruption
Global emergencies, such as COVID-19
in 2020, can easily disrupt supply chains. This disruption has a higher
chance of affecting large organizations - especially when there is only
a few large suppliers. Smaller organizations with robust, local supply
networks can manage supply chain shocks because any localized shock has a
smaller effect on the overall ecosystem.
Other effects which reduce competitiveness of large firms
These do not always increase the cost-per-unit, but do reduce the ability of a large firm to compete.
Cannibalization
A
small firm only competes with other firms, but larger firms frequently
find their own products are competing with each other. A Buick was just as likely to steal customers from another GM make, such as an Oldsmobile,
as it was to steal customers from other companies. This may help to
explain why Oldsmobiles were discontinued after 2004. This self-competition wastes resources that should be used to compete with other firms.
Isolation of decision-makers from the results of their decisions
If
a single person makes and sells donuts and decides to try jalapeño
flavoring, they would likely know on the same day whether their decision
was good or not, based on the reaction of customers. A decision-maker
at a huge company that makes donuts may not know for many months if such
a decision is embraced by consumers or if it is rejected, especially if
their research or marketing team fails to respond in a timely manner.
By that time, the decision-makers may very well have moved on to another
division or company and thus see no consequence from their decision.
This lack of consequences can lead to poor decisions and cause an
upward-sloping average cost curve.
Slow response time
In
a reverse example, the smaller firm will know immediately if people
begin to request other products, and be able to respond the next day. A
large company would need to do research, create an assembly line,
determine which distribution chains to use, plan an advertising
campaign, etc., before any changes could be made. By this time, the
smaller competitors may well have grabbed that market niche.
Inertia (Unwillingness to change)
This will be defined as the "we've always done it that way, so there's no need to ever change" attitude (see appeal to tradition).
An old, successful company is far more likely to have this attitude
than a new, struggling one. While "change for change's sake" is
counter-productive, refusal to consider change, even when indicated, is
likewise toxic to a company, as changes in the industry and market
conditions will inevitably demand changes in the firm in order to remain
successful. An example is Polaroid Corporation's delay in moving into digital imaging, which adversely affected the company, ultimately leading to bankruptcy.
Public and government opposition
Such opposition is largely a function of the size of the firm. Behavior from Microsoft, which would have been ignored from a smaller firm, was seen as an anti-competitive and monopolistic threat, due to Microsoft's size, thus bringing about government lawsuits.
A
small company with only a 1% market share could relatively easily
double market share, and hence revenues, in a year. A large company with
50% market share will find it difficult to do so.
Large market portfolio
A
small investment fund can potentially yield a higher return because it
can concentrate its investments in a small number of good opportunities
without driving up the purchase price as they buy in, and later sell
them without driving down the sale price as they sell off. Conversely, a
large investment fund must spread its investments among so many
securities that its results tend to track those of the market as a
whole. As the size of the market controlled grows, the results will be
closer to market average.
Inelasticity of supply
A
company which is heavily dependent on a resource supply of a fixed or
relatively-fixed size will have trouble increasing production. For
instance, a timber company cannot increase production above the
sustainable harvest rate of its land (although it can still increase
production by acquiring more land). Similarly, service companies are
limited by available labor (and thus tend to concentrate in large,
densely-populated metropolitan areas); STEM (science, technology, engineering, and mathematics) professions are often-cited examples.
Reputation
Larger
firms have a reputation to uphold and as a result may place more
restrictions on employees, limiting their efficiency. This will be seen
amplified in a regulated industry, where a company losing its license
would be an extremely serious event.
Large
firms also tend to be old and in mature markets. Both of these have
negative implications for future growth. Old firms tend to have a large
retiree base, with high associated pension and health costs, and also
tend to be unionized, with associated higher labor costs and lower productivity.
Mature markets tend to only offer the potential for small, incremental
growth. (Everybody might go out and buy a new invention next year, but
it is unlikely they will all buy cars next year, since most people
already have them.)
Impact on smaller firms
While
diseconomies of scale are typically associated with large mature firms,
similar problems have been observed in the growth phase of small and
medium-sized manufacturing companies. Mclean
has observed that this can occur once the workforce exceeds around 20
employees. At this point business complexity grows more rapidly than
revenue. The business experiences falling productivity, leading to
rising variable costs along with rapidly rising overheads.
Solutions
Solutions
to the diseconomies of scale for large firms may involve splitting the
company into smaller organisations. This can either happen by default
when the company is in financial difficulties, sells off its profitable
divisions and shuts down the rest; or can happen proactively, if the
management is willing.
To avoid the negative effects of diseconomies of scale, a firm
must stick to the lowest average output cost and try to recognise any
external diseconomies of scale. Moreover, on reaching the lowest average
cost, a firm must either expand to other countries to increase demand
for its products, or seek new markets or produce new products that do
not compete with its original products. However, neither of these
actions will necessarily eliminate communications and management
problems often associated with large organisations.
A systematic analysis and redesign of business processes,
in order to reduce complexity, can counter diseconomies of scale. (Of
course, this phase of analysis and revamping in itself can be, and
usually is, a diseconomy leading to hiring of new personnel and
investment in new, competing systems.) This leads to increased
productivity. Improved management systems and more effective control of
labor and operations can lower overhead.
Example
Returning
to the example of the large donut firm, each retail location could be
allowed to operate relatively autonomously from the company
headquarters.
For instance, the local management may decide on the following factors instead of relying on the central management:
- Employee decisions such as hiring, firing, promotions and wage scales, where the local management is directly involved and likely to have better understanding of each employee. For instance, employers may choose to offer higher wages and charge higher prices if they are in an affluent area.
- Purchasing decisions, with each location allowed to choose its own suppliers, which may or may not be owned by the corporation (wherever they find the best quality and prices).
- Research and marketing decisions. Each firm may decide to develop their own recipes or utilise different signature flavour unique to their locale. For instance, when fresh apple cider is available at bargain prices from local farmers in October, they may choose to market a cinnamon donut and hot apple cider combo.
While a single, large, centrally-controlled firm may have higher
ability to innovate and develop or market new products more effectively
than when its resources are divided, it may lack the flexibility to
offer individual customizations. Allowing the different retail locations
to make decisions independent of the central management may allow them
to meet local consumers' demands more efficiently.
In addition, if the employees own a portion of the local
business, employees will also have a more vested interest in its
success.
Note that all these changes will likely result in a substantial
reduction in corporate headquarters staff and other support staff. For
this reason, many businesses delay such a reorganization until it is too
late to be effective. However, the whole company incurs reputation and
legal risks arising from each unit.