Ebola virus disease
From Wikipedia, the free encyclopedia
| Ebola virus disease | |
|---|---|
| Classification and external resources | |

1976 photograph of two nurses standing in front of Mayinga N., a person with Ebola virus disease; she died only a few days later due to severe internal hemorrhaging.
| |
| ICD-10 | A98.4 |
| ICD-9 | 065.8 |
| DiseasesDB | 18043 |
| MedlinePlus | 001339 |
| eMedicine | med/626 |
| MeSH | D019142 |
Ebola virus disease (EVD) or Ebola hemorrhagic fever (EHF) is a disease of humans and other primates caused by an ebolavirus. Symptoms start two days to three weeks after contracting the virus, with a fever, sore throat, muscle pain, and headaches. Typically nausea, vomiting, and diarrhea follow, along with decreased functioning of the liver and kidneys. Around this time, affected people may begin to bleed both within the body and externally. [1]
The virus may be acquired upon contact with blood or bodily fluids of an infected animal (commonly monkeys or fruit bats).[1] Spread through the air has not been documented in the natural environment.[2] Fruit bats are believed to carry and spread the virus without being affected. Once human infection occurs, the disease may spread between people as well. Male survivors may be able to transmit the disease via semen for nearly two months. In order to make the diagnosis, typically other diseases with similar symptoms such as malaria, cholera, and other viral hemorrhagic fevers are first excluded. To confirm the diagnosis, blood samples are tested for viral antibodies, viral RNA, or the virus itself.[1]
Prevention includes decreasing the spread of disease from other infected animals to humans. This may be done by checking such animals for infection and killing and properly disposing of the bodies if the disease is discovered. Properly cooking meat and wearing protective clothing when handling meat may also be helpful, as are wearing protective clothing and washing hands when around a person with the disease. Samples of bodily fluids and tissues from people with the disease should be handled with special caution.[1]
There is no specific treatment for the disease.[1] Efforts to help those who are infected are supportive and include giving either oral rehydration therapy (slightly sweet and salty water to drink) or intravenous fluids.[1] The disease has a high mortality rate, often killing between 50% and 90% of those infected with the virus.[1][3] EVD was first identified in Sudan and the Democratic Republic of the Congo. The disease typically occurs in outbreaks in tropical regions of Sub-Saharan Africa.[1] From 1976 (when it was first identified) through 2013, fewer than 1,000 people per year have been infected.[1][4] The largest outbreak to date is the ongoing 2014 West Africa Ebola outbreak, which is affecting Guinea, Sierra Leone, Liberia, and Nigeria.[5][6] As of August 2014[update], more than 1,750 suspected cases have been reported.[7] Efforts are ongoing to develop a vaccine; however, none yet exists.[1]
Signs and symptoms
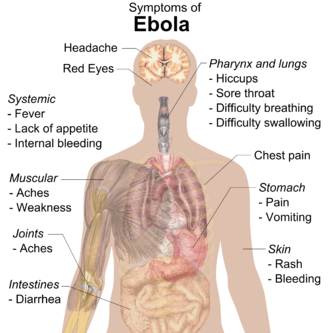
Symptoms of Ebola.[8]
Signs and symptoms of Ebola usually begin suddenly with a flu-like stage characterized by fatigue, fever, headaches, and joint, muscle, and abdominal pain.[9][10] Vomiting, diarrhea and loss of appetite are also common.[10] Less common symptoms include the following: sore throat, chest pain, hiccups, shortness of breath and trouble swallowing.[10] The average time between contracting the infection and the start of symptoms is 8 to 10 days, but it can vary between 2 and 21 days.[10] Skin manifestations may include a maculopapular rash (in about 50% of cases).[11] Early symptoms of EVD may be similar to those of malaria, dengue fever, or other tropical fevers, before the disease progresses to the bleeding phase.[9]
In 40–50% of cases, bleeding from puncture sites and mucous membranes (e.g. gastrointestinal tract, nose, vagina, and gums) has been reported.[12] In the bleeding phase, which typically starts 5 to 7 days after first symptoms[13] internal and subcutaneous bleeding may present itself through reddening of the eyes and bloody vomit.[9] Bleeding into the skin may create petechiae, purpura, ecchymoses, and hematomas (especially around needle injection sites). Types of bleeding known to occur with Ebola virus disease include vomiting blood, coughing it up or blood in the stool. Heavy bleeding is rare and is usually confined to the gastrointestinal tract.[11][14] In general, the development of bleeding symptoms often indicates a worse prognosis and this blood loss can result in death.[9] All people infected show some symptoms of circulatory system involvement, including impaired blood clotting.[11] If the infected person does not recover, death due to multiple organ dysfunction syndrome occurs within 7 to 16 days (usually between days 8 and 9) after first symptoms.[13]
Causes
EVD is caused by four of five viruses classified in the genus Ebolavirus, family Filoviridae, order Mononegavirales. The four disease-causing viruses are Bundibugyo virus (BDBV), Sudan virus (SUDV), Taï Forest virus (TAFV), and one called simply, Ebola virus (EBOV, formerly Zaire Ebola virus)). Ebola virus is the sole member of the Zaire ebolavirus species, and the most dangerous of the known Ebola disease causing viruses, as well as being responsible for the largest number of outbreaks.[15] The fifth virus, Reston virus (RESTV), is not thought to be disease-causing in humans.
The five Ebola viruses are closely related to the Marburg viruses.
Transmission
It is not entirely clear how Ebola is spread.[16] EVD is believed to occur after an ebola virus is transmitted to an initial human by contact with an infected animal's body fluids. Human-to-human transmission can occur via direct contact with blood or bodily fluids from an infected person (including embalming of an infected dead person) or by contact with contaminated medical equipment, particularly needles and syringes.[17] Semen is infectious in survivors for up to 50 days.Transmission through oral exposure and through conjunctiva exposure is likely[18] and has been confirmed in non-human primates.[19] The potential for widespread EVD infections is considered low as the disease is only spread by direct contact with the secretions from someone who is showing signs of infection.[17] The quick onset of symptoms makes it easier to identify sick individuals and limits a person's ability to spread the disease by traveling. Because dead bodies are still infectious, some doctors disposed of them in a safe manner, despite local traditional burial rituals.[20]
Medical workers who do not wear appropriate protective clothing may also contract the disease.[21] In the past, hospital-acquired transmission has occurred in African hospitals due to the reuse of needles and lack of universal precautions.[22][23]
Airborne transmission has not been documented during previous EVD outbreaks.[2] They are, however, infectious as breathable 0.8–1.2 micrometre laboratory generated droplets;[24] because of this potential route of infection, these viruses have been classified as Category A biological weapons.[25] Recently the virus has been shown to travel without contact from pigs to non-human primates, although the same study failed to achieve transmission in that manner between primates.[26]
Bats drop partially eaten fruits and pulp, then land mammals such as gorillas and duikers feed on these fallen fruits. This chain of events forms a possible indirect means of transmission from the natural host to animal populations, which has led to research towards viral shedding in the saliva of bats. Fruit production, animal behavior, and other factors vary at different times and places that may trigger outbreaks among animal populations.[27]
Reservoir
Bats are considered the most likely natural reservoir of the Ebola virus (EBOV); plants, arthropods, and birds have also been considered.[29] Bats were known to reside in the cotton factory in which the first cases for the 1976 and 1979 outbreaks were employed, and they have also been implicated in Marburg virus infections in 1975 and 1980.[30] Of 24 plant species and 19 vertebrate species experimentally inoculated with EBOV, only bats became infected.[31] The absence of clinical signs in these bats is characteristic of a reservoir species. In a 2002–2003 survey of 1,030 animals including 679 bats from Gabon and the Republic of the Congo, 13 fruit bats were found to contain EBOV RNA fragments.[32] As of 2005, three types of fruit bats (Hypsignathus monstrosus, Epomops franqueti, and Myonycteris torquata) have been identified as being in contact with EBOV. They are now suspected to represent the EBOV reservoir hosts.[33][34] Antibodies against Ebola Zaire and Reston viruses have been found in fruit bats in Bangladesh, thus identifying potential virus hosts and signs of the filoviruses in Asia.[35]
Between 1976 and 1998, in 30,000 mammals, birds, reptiles, amphibians, and arthropods sampled from outbreak regions, no ebolavirus was detected apart from some genetic traces found in six rodents (Mus setulosus and Praomys) and one shrew (Sylvisorex ollula) collected from the Central African Republic.[30][36] Traces of EBOV were detected in the carcasses of gorillas and chimpanzees during outbreaks in 2001 and 2003, which later became the source of human infections. However, the high lethality from infection in these species makes them unlikely as a natural reservoir.[30]
Transmission between natural reservoir and humans is rare, and outbreaks are usually traceable to a single case where an individual has handled the carcass of gorilla, chimpanzee, or duiker.[37] Fruit bats are also eaten by people in parts of West Africa where they are smoked, grilled, or made into a spicy soup.[34][38]
Virology
Genome
Like all mononegaviruses, ebolavirions contain linear nonsegmented, single-strand, non-infectious RNA genomes of negative polarity that possesses inverse-complementary 3' and 5' termini, do not possess a 5' cap, are not polyadenylated, and are not covalently linked to a protein.[39] Ebolavirus genomes are approximately 19 kilobase pairs long and contain seven genes in the order 3'-UTR-NP-VP35-VP40-GP-VP30-VP24-L-5'-UTR.[40] The genomes of the five different ebolaviruses (BDBV, EBOV, RESTV, SUDV, and TAFV) differ in sequence and the number and location of gene overlaps.
Structure
Like all filoviruses, ebolavirions are filamentous particles that may appear in the shape of a shepherd's crook or in the shape of a "U" or a "6", and they may be coiled, toroid, or branched.[40] In general, ebolavirions are 80 nm in width, but vary somewhat in length. In general, the median particle length of ebolaviruses ranges from 974 to 1,086 nm (in contrast to marburgvirions, whose median particle length was measured at 795–828 nm), but particles as long as 14,000 nm have been detected in tissue culture.[41]Replication
The ebolavirus life cycle begins with virion attachment to specific cell-surface receptors, followed by fusion of the virion envelope with cellular membranes and the concomitant release of the virus nucleocapsid into the cytosol. The viral RNA polymerase, encoded by the L gene, partially uncoats the nucleocapsid and transcribes the genes into positive-strand mRNAs, which are then translated into structural and nonstructural proteins. Ebolavirus RNA polymerase (L) binds to a single promoter located at the 3' end of the genome. Transcription either terminates after a gene or continues to the next gene downstream. This means that genes close to the 3' end of the genome are transcribed in the greatest abundance, whereas those toward the 5' end are least likely to be transcribed. The gene order is, therefore, a simple but effective form of transcriptional regulation. The most abundant protein produced is the nucleoprotein, whose concentration in the cell determines when L switches from gene transcription to genome replication. Replication results in full-length, positive-strand antigenomes that are, in turn, transcribed into negative-strand virus progeny genome copy. Newly synthesized structural proteins and genomes self-assemble and accumulate near the inside of the cell membrane.Virions bud off from the cell, gaining their envelopes from the cellular membrane they bud from. The mature progeny particles then infect other cells to repeat the cycle.The Ebola Virus genetics are difficult to study due to its virulent nature[42]
Pathophysiology
Endothelial cells, mononuclear phagocytes, and hepatocytes are the main targets of infection. After infection, a secreted glycoprotein (sGP) known as the Ebola virus glycoprotein (GP) is synthesized. Ebola replication overwhelms protein synthesis of infected cells and host immune defenses. The GP forms a trimeric complex, which binds the virus to the endothelial cells lining the interior surface of blood vessels. The sGP forms a dimeric protein that interferes with the signaling of neutrophils, a type of white blood cell, which allows the virus to evade the immune system by inhibiting early steps of neutrophil activation. These white blood cells also serve as carriers to transport the virus throughout the entire body to places such as the lymph nodes, liver, lungs, and spleen.[43]
The presence of viral particles and cell damage resulting from budding causes the release of cytokines (to be specific, TNF-α, IL-6, IL-8, etc.), which are the signaling molecules for fever and inflammation. The cytopathic effect, from infection in the endothelial cells, results in a loss of vascular integrity. This loss in vascular integrity is furthered with synthesis of GP, which reduces specific integrins responsible for cell adhesion to the inter-cellular structure, and damage to the liver, which leads to coagulopathy.[44]
Diagnosis
The medical history, especially travel and work history along with exposure to wildlife are important to suspect the diagnosis of EVD. The diagnosis is confirmed by isolating the virus, detecting its RNA or proteins, or detecting antibodies against the virus in a person's blood. Isolating the virus by cell culture, detecting the viral RNA by polymerase chain reaction (PCR) and detecting proteins by enzyme-linked immunosorbent assay (ELISA) is effective early and in those who have died from the disease. Detecting antibodies against the virus is effective late in the disease and in those who recover.[45]During an outbreak, virus isolation is often not feasible. The most common diagnostic methods are therefore real time PCR and ELISA detection of proteins, which can be performed in field or mobile hospitals.[46] Filovirions can be seen and identified in cell culture by electron microscopy due to their unique filamentous shapes, but electron microscopy cannot tell the difference between the various filoviruses despite there being some length differences.[41]

Phylogenetic tree comparing the Ebolavirus and Marburgvirus. Numbers indicate percent confidence of branches.
Classification
The genera Ebolavirus and Marburgvirus were originally classified as the species of the now-obsolete Filovirus genus. In March 1998, the Vertebrate Virus Subcommittee proposed in the International Committee on Taxonomy of Viruses (ICTV) to change the Filovirus genus to the Filoviridae family with two specific genera: Ebola-like viruses and Marburg-like viruses. This proposal was implemented in Washington, DC, on April 2001 and in Paris on July 2002. In 2000, another proposal was made in Washington, D.C., to change the "-like viruses" to "-virus" resulting in today's Ebolavirus and Marburgvirus.[47]Rates of genetic change are 100 times slower than influenza A in humans, but on the same magnitude as those of hepatitis B. Extrapolating backwards using these rates indicates that Ebolavirus and Marburgvirus diverged several thousand years ago.[48] However, paleoviruses (genomic fossils) of filoviruses (Filoviridae) found in mammals indicate that the family itself is at least tens of millions of years old.[49] Fossilized viruses that are closely related to ebolaviruses have been found in the genome of the Chinese hamster.[50]
Differential diagnosis
The symptoms of EVD are similar to those of Marburg virus disease.[51] It can also easily be confused with many other diseases common in Equatorial Africa such as other viral hemorrhagic fevers, falciparum malaria, typhoid fever, shigellosis, rickettsial diseases such as typhus, cholera, gram-negative septicemia, borreliosis such as relapsing fever or EHEC enteritis. Other infectious diseases that should be included in the differential diagnosis include the following: leptospirosis, scrub typhus, plague, Q fever, candidiasis, histoplasmosis, trypanosomiasis, visceral leishmaniasis, hemorrhagic smallpox, measles, and fulminant viral hepatitis.[citation needed] Non-infectious diseases that can be confused with EVD are acute promyelocytic leukemia, hemolytic uremic syndrome, snake envenomation, clotting factor deficiencies/platelet disorders, thrombotic thrombocytopenic purpura, hereditary hemorrhagic telangiectasia, Kawasaki disease, and even warfarin poisoning.[52][53][54][55]Prevention

A researcher working with the Ebola virus while wearing a BSL-4 positive pressure suit to avoid infection
Behavioral changes
Ebola viruses are contagious, with prevention predominantly involving behavior changes, proper full-body personal protective equipment, and disinfection. Techniques to avoid infection involve not contacting infected blood or secretions, including from those who are dead.[16] This involves suspecting and diagnosing the disease early and using standard precautions for all patients in the healthcare setting.[56] Recommended measures when caring for those who are infected include isolating them, sterilizing equipment, and wearing protective clothing including masks, gloves, gowns, and goggles.[16] Hand washing is important but can be difficult in areas where there is not even enough water for drinking.[9]Due to lack of proper equipment and hygienic practices, large-scale epidemics have occurred mostly in poor, isolated areas without modern hospitals or well-educated medical staff. Traditional burial rituals, especially those requiring embalming of bodies, should be discouraged or modified.[56] Airline crews, who fly to these areas of the world, are taught to identify Ebola and isolate anyone who has symptoms.[57]
Quarantine
Quarantine, also known as enforced isolation, is usually effective in decreasing spread.[58][59] Governments often quarantine areas where the disease is occurring or individuals who may be infected.[60] In the United States, the law allows quarantine of those infected with Ebola.[61] The lack of roads and transportation may help slow the disease in Africa. During the 2014 outbreak, Liberia closed schools.[62]Vaccine
No vaccine is currently available for humans.[1][63][64] The most promising candidates are DNA vaccines[65] or vaccines derived from adenoviruses,[66] vesicular stomatitis Indiana virus (VSIV)[67][68][69] or filovirus-like particles (VLPs)[70] because these candidates could protect nonhuman primates from ebolavirus-induced disease. DNA vaccines, adenovirus-based vaccines, and VSIV-based vaccines have entered clinical trials.[71][72][73][74]Vaccines have protected nonhuman primates. Immunization takes six months, which impedes the counter-epidemic use of the vaccines. Searching for a quicker onset of effectiveness, in 2003, a vaccine using an adenoviral (ADV) vector carrying the Ebola spike protein was tested on crab-eating macaques. Twenty-eight days later, they were challenged with the virus and remained resistant.[66] A vaccine based on attenuated recombinant vesicular stomatitis virus (VSV) vector carrying either the Ebola glycoprotein or the Marburg glycoprotein in 2005 protected nonhuman primates,[75] opening clinical trials in humans.[71] The study by October completed the first human trial, over three months giving three vaccinations safely inducing an immune response. Individuals for a year were followed, and, in 2006, a study testing a faster-acting, single-shot vaccine began; this new study was completed in 2008.[72] Trying the vaccine on a strain of Ebola that more resembles one that infects humans is the next step.[citation needed]
On 6 December 2011, the development of a successful vaccine against Ebola for mice was reported. Unlike the predecessors, it can be freeze-dried and thus stored for long periods in wait for an outbreak.[76] An experimental vaccine made by researchers at Canada's national laboratory in Winnipeg was used, in 2009, to pre-emptively treat a German scientist who might have been infected during a lab accident.[77] However, actual EBOV infection could never be demonstrated without a doubt.[78] Experimentally, recombinant vesicular stomatitis Indiana virus (VSIV) expressing the glycoprotein of EBOV or SUDV has been used successfully in nonhuman primate models as post-exposure prophylaxis.[79][80][clarification needed]
Laboratory
Ebola viruses are World Health Organization Risk Group 4 pathogens, requiring biosafety level 4-equivalent containment. Laboratory researchers must be properly trained in BSL-4 practices and wear proper personal protective equipment.Treatment
No ebolavirus-specific treatment exists.[64] Treatment is primarily supportive in nature and includes minimizing invasive procedures, balancing fluids and electrolytes to counter dehydration, administration of anticoagulants early in infection to prevent or control disseminated intravascular coagulation, administration of procoagulants late in infection to control bleeding, maintaining oxygen levels, pain management, and the use of medications to treat bacterial or fungal secondary infections.[81][82][83] Early treatment may increase the chance of survival.[84] A number of experimental treatments are being studied.[85]
In the United States, the FDA's animal efficacy rule can be used in combination with a phase I clinical trial to demonstrate reasonable safety for an experimental, unapproved drug, to obtain permission to treat people who are infected with Ebola with the drug under the Expanded access program. The Animal Efficacy Rule exists, because the normal path for testing the safety and efficacy of drugs is not possible for diseases caused by dangerous pathogens or toxins.[86] The FDA allowed two drugs, ZMapp and an RNA interference drug called "TKM-Ebola", to be used in people infected with Ebola under these programs during the 2014 outbreak.[87]
The unavailability of experimental treatments in the most affected regions during the 2014 outbreak spurred controversy, with some calling for experimental drugs to be made more widely available in Africa on a humanitarian basis, and others warning that making unproven experimental drugs widely available would be unethical, especially in light of past experimentation conducted in developing countries by Western drug companies.[88][89] As a result of the controversy, the World Health Organization convened an expert meeting of bioethicists on 11 August 2014 to consider the implications of making the experimental treatment more widely available.[90] The panel reached consensus that the circumstances warrant the use of unproven interventions with as yet unknown efficacy and adverse effects both for treatment and for prevention, and also said that deciding which treatments should be used and how to distribute them equitably were matters that needed further discussion.[91]
Prognosis
The disease has a high mortality rate: often between 50 percent and 90 percent.[1][3] As of April 2014[update], information from WHO across all occurrences to date puts the overall fatality rate at 60%-65%.[1]There are indications based on variations in death rate between countries that early and effective treatment of symptoms (e.g., supportive care to prevent dehydration) may reduce the fatality rate significantly.[92] If an infected person survives, recovery may be quick and complete. Prolonged cases are often complicated by the occurrence of long-term problems, such as inflammation of the testicles, joint pains, muscle pains, skin peeling, or hair loss. Eye symptoms, such as light sensitivity, excess tearing, iritis, iridocyclitis, choroiditis, and blindness have also been described. EBOV and SUDV may be able to persist in the semen of some survivors for up to seven weeks, which could give rise to infections and disease via sexual intercourse.[1]
Epidemiology
The disease typically occurs in outbreaks in tropical regions of Sub-Saharan Africa.[1] From 1976 (when it was first identified) through 2013, the World Health Organization reported 1,716 confirmed cases.[1][4] The largest outbreak to date is the ongoing 2014 West Africa Ebola virus outbreak, which is affecting Guinea, Sierra Leone,Liberia and Nigeria[5][6] As of 13 August, 2,127 cases have been identified, with 1,145 deaths.[5]1976
The first identified case of Ebola was on 26 August 1976, in Yambuku, a small rural village in Mongala District in northern Democratic Republic of the Congo (DRC, then known as Zaire).[93] The first victim, and the index case for the disease, was village school headmaster Mabalo Lokela, who had toured an area near the Central African Republic border along the Ebola river between 12-22 August. On 8 September he died of what would become known as the Ebola virus species of the ebolavirus.[94] Subsequently a number of other cases were reported, almost all centered on the Yambuku mission hospital or having close contact with another case.[95] 318 cases and 280 deaths occurred in the DRC. The Ebola outbreak was contained with the help of the World Health Organization and transport from the Congolese air force, by quarantining villagers, sterilizing medical equipment, and providing protective clothing. The virus responsible for the initial outbreak, first thought to be Marburg virus was later identified as a new type of virus related to Marburg, and named after the nearby Ebola river. Another ebolavirus, the Sudan virus species, was also identified that same year when an outbreak occurred in Sudan, affecting 284 people and killing 151.[96]1995 to 2013
The second major outbreak occurred in 1995 in the Democratic Republic of Congo, affecting 315 and killing 254. The next major outbreak occurred in Uganda in 2000, affecting 425 and killing 224; in this case the Sudan virus was found to be the ebolavirus species responsible for the outbreak. .[97] In 2003 there was an outbreak in the Republic of Congo that affected 143 and killed 128, a death rate of 90%, the largest to date.[98]In August 2007, 103 people were infected by a suspected hemorrhagic fever outbreak in the village of Kampungu, Democratic Republic of the Congo. The outbreak started after the funerals of two village chiefs, and 217 people in four villages fell ill. .[99][100] [101] The 2007 outbreak eventually affected 264 individuals and resulted in the deaths of 187 .[1]
On 30 November 2007, the Uganda Ministry of Health confirmed an outbreak of Ebola in the Bundibugyo District in Western Uganda. After confirmation of samples tested by the United States National Reference Laboratories and the Centers for Disease Control, the World Health Organization confirmed the presence of a new species of Ebolavirus, which was tentatively named Bundibugyo.[102] The WHO reported 149 cases of this new strain and 37 of those led to deaths.[1]
The Who confirmed two small outbreaks in Uganda in 2012. The first outbreak affected 7 people and resulted in the death of 4 and the second affected 24, resulting in the death of 17. The Sudan variant was responsible for both outbreaks.[1]
On 17 August 2012, the Ministry of Health of the Democratic Republic of the Congo reported an outbreak of the Ebola-Bundibugyo variant[103] in the eastern region.[104] [105] Other than its discovery in 2007, this was the only time that this variant has been identified as the ebolavirus responsible for an outbreak. The WHO revealed that the virus had sickened 57 people and claimed 29 lives. The probable cause of the outbreak was tainted bush meat hunted by local villagers around the towns of Isiro and Viadana.[106][1]
2014 outbreak
In March 2014, the World Health Organization (WHO) reported a major Ebola outbreak in Guinea, a western African nation; it is the largest ever documented, and the first recorded in the region.[107]
Researchers traced the outbreak to a two-year old child who died on 6 December.[108] As of 10 April 2014, WHO reported 157 suspected and confirmed cases in Guinea, 22 suspected cases in Liberia, and 8 suspected cases in Sierra Leone.[109][110] By 31 July 2014, they reported that the death toll had reached 826 people from 1440 cases.[111] On 8 August, the WHO declared the epidemic to be an international public health emergency. Urging the world to offer aid to the affected regions, the Director-General said, "Countries affected to date simply do not have the capacity to manage an outbreak of this size and complexity on their own. I urge the international community to provide this support on the most urgent basis possible."[112][113] Further attempts to contain the outbreak were enacted by placing troops on roads to cordon off the infected areas and stop those who may be infected from leaving and further spreading the virus.[114]
Emory University Hospital was the first US hospital to care for people exposed to Ebola.[115] In July 2014, two American medical providers were exposed while treating infected patients in Liberia. In August, arrangements were made for them to be transported to Emory via speciality aircraft. Emory Hospital has a specially built isolation unit set up in collaboration with the CDC to treat people exposed to certain serious infectious diseases.[116][117][118][119]
History
Ebola virus was first isolated in 1976 during outbreaks of Ebola hemorrhagic fever in the Democratic Republic of the Congo (then Zaire)[120] and Sudan.[121] The strain of Ebola that broke out in the Democratic Republic of the Congo had one of the highest case fatality rates of any human virus, 88%.[122]
The name of the disease originates from the first recorded outbreak in 1976 in Yambuku, Democratic Republic of the Congo, which lies on the Ebola River.[120]
In late 1989, Hazelton Research Products' Reston Quarantine Unit in Reston, Virginia, suffered a mysterious outbreak of fatal illness (initially diagnosed as Simian hemorrhagic fever virus (SHFV)) among a shipment of crab-eating macaque monkeys imported from the Philippines. Hazelton's veterinary pathologist sent tissue samples from dead animals to the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) at Fort Detrick, Maryland, where a laboratory test known as an ELISA assay showed antibodies to Ebola virus.[123] An electron microscopist from USAMRIID discovered filoviruses similar in appearance to Ebola in tissue samples taken from crab-eating macaque imported from the Philippines to Hazleton Laboratories Reston, Virginia.[124]
Shortly afterward, a US Army team headquartered at USAMRIID went into action to euthanize the monkeys which had not yet died, bringing those monkeys and those which had already died of the disease to Ft. Detrick for study by the Army's veterinary pathologists and virologists, and eventual disposal under safe conditions.[123]
Blood samples were taken from 178 animal handlers during the incident.[125] Of those, six animal handlers eventually seroconverted. When the handlers did not become ill, the CDC concluded that the virus had a very low pathogenicity to humans.[126]
The Philippines and the United States had no previous cases of Ebola infection, and upon further isolation, researchers concluded it was another strain of Ebola, or a new filovirus of Asian origin, which they named Reston ebolavirus (REBOV) after the location of the incident.[123]
Society and culture
Given the lethal nature of Ebola, and since no approved vaccine or treatment is available, it is classified as a biosafety level 4 agent, as well as a Category A bioterrorism agent by the Centers for Disease Control and Prevention. It has the potential to be weaponized for use in biological warfare.[127][128]Other animals
It is widely believed that outbreaks of EVD among human populations result from handling infected wild animal carcasses. Some research suggests that an outbreak in the wild animals used for consumption (bush meat) may result in a corresponding human outbreak. Since 2003, such outbreaks have been monitored through surveillance of animal populations with the aim of predicting and preventing Ebola outbreaks in humans.[129]Recovered carcasses from gorillas contain multiple Ebola virus strains, which suggest multiple introductions of the virus. Bodies decompose quickly and carcasses are not infectious after three to four days. Contact between gorilla groups is rare, suggesting transmission among gorilla groups is unlikely, and that outbreaks result from transmission between viral reservoir and animal populations.[130]
Ebola has a high mortality among primates.[131] Frequent outbreaks of Ebola may have resulted in the deaths of 5,000 gorillas.[132] Outbreaks of Ebola may have been responsible for an 88% decline in tracking indices of observed chimpanzee populations in 420 square kilometer Lossi Sanctuary between 2002 and 2003.[130] Transmission among chimpanzees through meat consumption constitutes a significant risk factor, while contact between individuals, such as touching dead bodies and grooming, is not.[133]
Domestic animals
Ebola virus can be transmitted to dogs and pigs.[134] While dogs may be asymptomatic, pigs tend to develop symptomatic disease.Research
Medications
Favipiravir looks like it may be useful in a mouse model of the disease.[9] Estrogen receptor drugs used to treat infertility and breast cancer (clomiphene and toremifene) inhibit the progress of Ebola virus in infected mice.[135] Ninety percent of the mice treated with clomiphene and fifty percent of those treated with toremifene survived the tests.[135] A 2014 study found that Amiodarone, an ion channel blocker used in the treatment of heart arrhythmias, blocks the entry of ebola virus into cells in vitro.[136] Given their oral availability and history of human use, these drugs would be candidates for treating Ebola virus infection in remote geographical locations, either on their own or together with other antiviral drugs.Antibodies
During an outbreak 1999 in the Democratic Republic of the Congo, seven of eight people who received blood transfusions from individuals who had previously survived the infection survived themselves.[137] However, this potential treatment is considered controversial.[138] Intravenous antibodies appear to be protective in non-human primates who have been exposed to large doses of Ebola.[139]
Use of untested experimental drugs
On 31 July 2014, an experimental drug, ZMapp, was first tested on humans. It was administered to two Americans who had been infected with Ebola. Both people appeared to have had positive results.[140][141] Soon thereafter ZMapp was administered to a third Ebola patient, a 75 year old priest, who nonetheless died.[142]On 12 August the WHO released an advisory statement regarding the ongoing Ebola outbreak, recommending specifically for the more widespread use of the compassionate use exemption for experimental drugs, in efforts to contain the ongoing Ebola outbreak, with the aim of more quickly halting and eliminating the current Ebola outbreak.[143]