Neuron and myelinated axon, with signal flow from inputs at dendrites to outputs at axon terminals
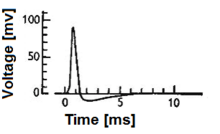
Fig
1. A neuronal action potential ("spike"). Note that the amplitude and
the exact shape of the action potential can vary according to the exact
experimental technique used for acquiring the signal.
A biological neuron model, also known as a spiking neuron model,
is a mathematical description of the properties of certain cells in the
nervous system that generate sharp electrical potentials across their cell membrane, roughly one millisecond in duration, as shown in Fig. 1. Spiking neurons are known to be a major signaling unit of the nervous system,
and for this reason characterizing their operation is of great
importance. It is worth noting that not all the cells of the nervous
system produce the type of spike that define the scope of the spiking
neuron models. For example, cochlear hair cells, retinal receptor cells, and retinal bipolar cells do not spike. Furthermore, many cells in the nervous system are not classified as neurons but instead are classified as glia.
Fig
2. "Whole cell" measurement technique, which captures the spiking
activity of a single neuron and produces full amplitude action
potentials.
Ultimately, biological neuron models aim to explain the mechanisms
underlying the operation of the nervous system for the purpose of
restoring lost control capabilities such as perception (e.g. deafness or blindness), motor movement decision making, and continuous limb control. In that sense, biological neuron models differ from artificial neuron
models that do not presume to predict the outcomes of experiments
involving the biological neural tissue (although artificial neuron
models are also concerned with execution of perception and estimation
tasks). Accordingly, an important aspect of biological neuron models is
experimental validation, and the use of physical units to describe the
experimental procedure associated with the model predictions.
Neuron models can be divided into two categories according to the
physical units of the interface of the model. Each category could be
further divided according to the abstraction/detail level:
- Electrical input–output membrane voltage models – These models produce a prediction for membrane output voltage as function of electrical stimulation at the input stage (either voltage or current). The various models in this category differ in the exact functional relationship between the input current and the output voltage and in the level of details. Some models in this category are black box models and distinguish only between two measured voltage levels: the presence of a spike (also known as "action potential") or a quiescent state. Other models are more detailed and account for sub-cellular processes.
- Natural or pharmacological input neuron models – The models in this category connect between the input stimulus which can be either pharmacological or natural, to the probability of a spike event. The input stage of these models is not electrical, but rather has either pharmacological (chemical) concentration units, or physical units that characterize an external stimulus such as light, sound or other forms of physical pressure. Furthermore, the output stage represents the probability of a spike event and not an electrical voltage. Typically, this output probability is normalized (divided by) a time constant, and the resulting normalized probability is called the "firing rate" and has units of Hertz. The probabilistic description taken by the models in this category was inspired from laboratory experiments involving either natural or pharmacological stimulation which exhibit variability in the resulting spike pattern. Nevertheless, when averaging these experimental results across several trials, a clear pattern is often revealed.
Fig
3. Extracellular measurement: Captures spikes with lower amplitudes,
often from several spiking sources, depending on the size of the
electrode and its proximity to the sources. Despite the decreased
amplitude levels produced by this technique, it also has several
advantages: 1) Is easier to obtain experimentally; 2) Is robust and
lasts for a longer time; 3) Can reflect the dominant effect, especially
when conducted in an anatomical region with many similar cells.
Although it is not unusual in science and engineering to have several
descriptive models for different abstraction/detail levels, the number
of different, sometimes contradicting, biological neuron models is
exceptionally high. This situation is partly the result of the many
different experimental settings, and the difficulty to separate the
intrinsic properties of a single neuron from measurements effects and
interactions of many cells. To accelerate the convergence to a unified theory, we list
several models in each category, and where applicable, also references
to supporting experiments.
Electrical input–output membrane voltage models
The
models in this category describe the relationship between neuronal
membrane currents at the input stage, and membrane voltage at the output
stage. The most extensive experimental inquiry in this category of
models was made by Hodgkin–Huxley in the early 1950s using an
experimental setup that punctured the cell membrane and allowed to force
a specific membrane voltage/current.
Most modern electrical neural interfaces
apply extra-cellular electrical stimulation to avoid membrane
puncturing which can lead to cell death and tissue damage. Hence, it is
not clear to what extent the electrical neuron models hold for
extra-cellular stimulation.
Integrate-and-fire
One of the earliest models of a neuron was first investigated in 1907 by Louis Lapicque. A neuron is represented in time by
which is just the time derivative of the law of capacitance, Q = CV. When an input current is applied, the membrane voltage increases with time until it reaches a constant threshold Vth, at which point a delta function spike occurs and the voltage is reset to its resting potential, after which the model continues to run. The firing frequency of the model thus increases linearly without bound as input current increases.
The model can be made more accurate by introducing a refractory period tref
that limits the firing frequency of a neuron by preventing it from
firing during that period. Through some calculus involving a Fourier transform, the firing frequency as a function of a constant input current thus looks like
- .
A remaining shortcoming of this model is that it implements no
time-dependent memory. If the model receives a below-threshold signal at
some time, it will retain that voltage boost forever until it fires
again. This characteristic is clearly not in line with observed neuronal
behavior.
Hodgkin–Huxley model
The Hodgkin–Huxley model (H andH model)
is a model of the relationship between ion currents crossing the neuronal cell membrane and the membrane voltage.
The model is based on experiments that allowed to force membrane
voltage using an intra-cellular pipette. This model is based on the
concept of membrane ion channels and relies on data from the squid giant axon. Hodgkin and Huxley were awarded the 1963 Nobel Prize in Physiology or Medicine for this model.
We note as before our voltage-current relationship, this time generalized to include multiple voltage-dependent currents:
- .
Each current is given by Ohm's Law as
where g(t,V) is the conductance, or inverse resistance, which can be expanded in terms of its constant average ḡ and the activation and inactivation fractions m and h, respectively, that determine how many ions can flow through available membrane channels. This expansion is given by
and our fractions follow the first-order kinetics
with similar dynamics for h, where we can use either τ and m∞ or α and β to define our gate fractions.
With such a form, all that remains is to individually investigate
each current one wants to include. Typically, these include inward Ca2+ and Na+ input currents and several varieties of K+ outward currents, including a "leak" current.
The end result can be at the small end 20 parameters which one
must estimate or measure for an accurate model, and for complex systems
of neurons not easily tractable by computer. Careful simplifications of
the Hodgkin–Huxley model are therefore needed.
Leaky integrate-and-fire
In
the leaky integrate-and-fire model, the memory problem is solved by
adding a "leak" term to the membrane potential, reflecting the diffusion
of ions that occurs through the membrane when some equilibrium is not
reached in the cell. The model looks like
where Rm is the
membrane resistance, as we find it is not a perfect insulator as assumed
previously. This forces the input current to exceed some threshold Ith = Vth / Rm in order to cause the cell to fire, else it will simply leak out any change in potential. The firing frequency thus looks like
which converges for large input currents to the previous leak-free model with refractory period.
Fractional-Order Leaky integrate-and-fire
Recent
advances in computational and theoretical fractional calculus lead to a
new form of model, called Fractional-Order Leaky integrate-and-fire
developed by Teka et al. The great advantage of this model is that it
can capture and integrate all the past activities and can reproduce the
time dependent spiking adaptations observed on pyramidal neurons. The
model has the following form more details can be found in
Galves-Löcherbach
3D
visualization of the Galves-Löcherbach model for biological neural
nets. This visualization is set for 4,000 neurons (4 layers with one
population of inhibitory neurons and one population of excitatory
neurons each) at 180 intervals of time.
The Galves-Löcherbach model is a specific development of the leaky integrate-and-fire model. It is inherently stochastic. It was developed by mathematicians Antonio Galves and Eva Löcherbach.
Given the model specifications, the probability that a given neuron spikes in a time period may be described by
where is a synaptic weight, describing the influence of neuron on neuron , expresses the leak, and provides the spiking history of neuron before , according to
Exponential integrate-and-fire
In the Exponential Integrate-and-Fire, spike generation is exponential, following the equation:
- .
where is the membrane potential, is the membrane potential threshold, is the membrane time constant, is the resting potential, and
is the sharpness of action potential initiation, usually around 1 mV
for cortical pyramidal neurons. Once the membrane potential crosses , it diverges to infinity in finite time.
FitzHugh–Nagumo
Sweeping simplifications to Hodgkin–Huxley were introduced by
FitzHugh and Nagumo in 1961 and 1962. Seeking to describe "regenerative
self-excitation" by a nonlinear positive-feedback membrane voltage and
recovery by a linear negative-feedback gate voltage, they developed the
model described by
where we again have a membrane-like voltage and input current with a slower general gate voltage w and experimentally-determined parameters a = -0.7, b = 0.8, τ = 1/0.08.
Although not clearly derivable from biology, the model allows for a
simplified, immediately available dynamic, without being a trivial
simplification.
Morris–Lecar
In 1981 Morris and Lecar combined Hodgkin–Huxley and FitzHugh–Nagumo
into a voltage-gated calcium channel model with a delayed-rectifier
potassium channel, represented by
where .
Hindmarsh–Rose
Building upon the FitzHugh–Nagumo model, Hindmarsh and Rose proposed
in 1984 a model of neuronal activity described by three coupled first
order differential equations:
with r2 = x2 + y2 + z2, and r ≈ 10−2 so that the z
variable only changes very slowly. This extra mathematical complexity
allows a great variety of dynamic behaviors for the membrane potential,
described by the x variable of the
model, which include chaotic dynamics. This makes the Hindmarsh–Rose
neuron model very useful, because being still simple, allows a good
qualitative description of the many different patterns of the action
potential observed in experiments.
Cable theory
Cable theory describes the dendritic arbor as a cylindrical structure undergoing a regular pattern of bifurcation,
like branches in a tree. For a single cylinder or an entire tree, the
input conductance at the base (where the tree meets the cell body, or
any such boundary) is defined as
- ,
where L is the electrotonic
length of the cylinder which depends on its length, diameter, and
resistance. A simple recursive algorithm scales linearly with the number
of branches and can be used to calculate the effective conductance of
the tree. This is given by
where AD = πld is the total surface area of the tree of total length l, and LD is its total electrotonic length. For an entire neuron in which the cell body conductance is GS and the membrane conductance per unit area is Gmd = Gm / A, we find the total neuron conductance GN for n dendrite trees by adding up all tree and soma conductances, given by
- ,
where we can find the general correction factor Fdga experimentally by noting GD = GmdADFdga.
Compartmental models
The cable model makes a number of simplifications to give closed
analytic results, namely that the dendritic arbor must branch in
diminishing pairs in a fixed pattern. A compartmental model allows for
any desired tree topology with arbitrary branches and lengths, but makes
simplifications in the interactions between branches to compensate.
Thus, the two models give complementary results, neither of which is
necessarily more accurate.
Each individual piece, or compartment, of a dendrite is modeled by a straight cylinder of arbitrary length l and diameter d which connects with fixed resistance to any number of branching cylinders. We define the conductance ratio of the ith cylinder as Bi = Gi / G∞, where and Ri
is the resistance between the current compartment and the next. We
obtain a series of equations for conductance ratios in and out of a
compartment by making corrections to the normal dynamic Bout,i = Bin,i+1, as
where the last equation deals with parents and daughters at branches, and .
We can iterate these equations through the tree until we get the point
where the dendrites connect to the cell body (soma), where the
conductance ratio is Bin,stem. Then our total neuron conductance is given by
- .
An example of a compartmental model of a neuron, with an algorithm to
reduce the number of compartments (increase the computational speed)
and yet retain the salient electrical characteristics, can be found in.
Natural input stimulus neuron models
The
models in this category were derived following experiments involving
natural stimulation such as light, sound, touch, or odor. In these
experiments, the spike pattern resulting from each stimulus presentation
varies from trial to trial, but the averaged response from several
trials often converges to a clear pattern. Consequently, the models in
this category generate a probabilistic relationship between the input
stimulus to spike occurrences.
The non-homogeneous Poisson process model (Siebert)
Siebert modeled the neuron spike firing pattern using a non-homogeneous Poisson process model, following experiments involving the auditory system. According to Siebert, the probability of a spiking event at the time interval is proportional to a non negative function , where is the raw stimulus.:
Siebert considered several functions as , including for low stimulus intensities.
The main advantage of Siebert's model is its simplicity. The
shortcomings of the model is its inability to reflect properly the
following phenomena:
- The edge emphasizing property of the neuron in response to a stimulus pulse.
- The saturation of the firing rate.
- The values of inter-spike-interval-histogram at short intervals values (close to zero).
These shortcoming are addressed by the two state Markov Model.
The two state Markov model (Nossenson and Messer)
The spiking neuron model by Nossenson and Messer produces the probability of the neuron to fire a spike as a function of either an external or pharmacological stimulus.
The model consists of a cascade of a receptor layer model and a spiking
neuron model, as shown in Fig 4. The connection between the external
stimulus to the spiking probability is made in two steps: First, a
receptor cell model translates the raw external stimulus to
neurotransmitter concentration, then, a spiking neuron model connects
between neurotransmitter concentration to the firing rate (spiking
probability). Thus, the spiking neuron model by itself depends on
neurotransmitter concentration at the input stage.
Fig 4: High level block diagram of the receptor layer and neuron model by Nossenson and Messer.
Fig 5. The prediction for the firing rate in response to a pulse stimulus as given by the model by Nossenson and Messer.
An important feature of this model is the prediction for neurons
firing rate pattern which captures, using a low number of free
parameters, the characteristic edge emphasized response of neurons to a
stimulus pulse, as shown in Fig. 5. The firing rate is identified both
as a normalized probability for neural spike firing, and as a quantity
proportional to the current of neurotransmitters released by the cell.
The expression for the firing rate takes the following form:
where,
- P0 is the probability of the neuron to be "armed" and ready to fire. It is given by the following differential equation:
- P0 could be generally calculated recursively using Euler method,
but in the case of a pulse of stimulus it yields a simple closed form
expression.
- y(t) is the input of the model and is interpreted as the neurotransmitter concentration on the cell surrounding (in most cases glutamate) . For an external stimulus it can be estimated through the receptor layer model:
- , with being short temporal average of stimulus power (given in Watt or other energy per time unit).
- R0 corresponds to the intrinsic spontaneous firing rate of the neuron.
- R1 is the recovery rate of the neuron from refractory state.
Other predictions by this model include:
1) The averaged Evoked Response Potential (ERP) due to population
of many neurons in unfiltered measurements resembles the firing rate.
2) The voltage variance of activity due to multiple neuron
activity resembles the firing rate (also known as Multi-Unit-Activity
power or MUA).
3) The inter-spike-interval probability distribution takes the form a gamma-distribution like function.
Non-Markovian models
The following is a list of published non-Markovian neuron models:
- Johnson, and Swami
- Berry and Meister
- Kass and Ventura
Theta model
The theta model, or Ermentrout–Kopell canonical model, is a model originally developed to model neurons in the animal Aplysia, and is particularly well suited to describe neuron parabolic bursting.
Pharmacological input stimulus neuron models
The models in this category produce predictions for experiments involving pharmacological stimulation.
Synaptic transmission (Koch and Segev)
According to the model by Koch and Segev,
the response of a neuron to individual neurotransmitters can be modeled
as an extension of the classical Hodgkin–Huxley model with both
standard and nonstandard kinetic currents. Four neurotransmitters
primarily have influence in the CNS. AMPA/kainate receptors are fast excitatory mediators while NMDA receptors mediate considerably slower currents. Fast inhibitory currents go through GABAA receptors, while GABAB receptors mediate by secondary G-protein-activated potassium channels. This range of mediation produces the following current dynamics:
where ḡ is the maximal conductance (around 1S) and E is the equilibrium potential of the given ion or transmitter (AMDA, NMDA, Cl, or K), while [O] describes the fraction of receptors that are open. For NMDA, there is a significant effect of magnesium block that depends sigmoidally on the concentration of intracellular magnesium by B(V). For GABAB, [G] is the concentration of the G-protein, and Kd describes the dissociation of G in binding to the potassium gates.
The dynamics of this more complicated model have been
well-studied experimentally and produce important results in terms of
very quick synaptic potentiation and depression, that is, fast, short-term learning.
The two state Markov model (Nossenson and Messer)
The
model by Nossenson and Messer translates neurotransmitter concentration
at the input stage to the probability of releasing neurotransmitter at
the output stage. For a more detailed description of this model, see the Two state Markov model section above.
HTM Neuron Model
The HTM Neuron Model was developed by Jeff Hawkins and researchers at Numenta and is based on a theory called Hierarchical Temporal Memory which was originally described in the book On Intelligence. It is based on neuroscience and the physiology and interaction of pyramidal neurons in the neocortex of the human brain.
Comparing the artificial neural network (A), the biological neuron (B), and the HTM neuron (C).
| - Few synapses
- No dendrites
- Sum input x weights - Learns by modifying weights of synapses |
- Thousands of synapses on the dendrites
- Active dendrites: cell recognizes hundreds of unique patterns
- Co-activation of a set of synapses on a dendritic segment causes an NMDA spike and depolarization at the soma - Sources of input to the cell:
|
- Inspired by the pyramidal cells in neocortex layers 2/3 and 5
- Thousands of synapses
- Active dendrites: cell recognizes hundreds of unique patterns - Models dendrites and NMDA spikes with each array of coincident detectors having a set of synapses - Learns by modeling growth of new synapses |
Relation between artificial and biological neuron models
The most basic model of a neuron consists of an input with some synaptic weight vector and an activation function or transfer function inside the neuron determining output. This is the basic structure used for artificial neurons, which in a neural network often looks like
where yi is the output of the i th neuron, xj is the jth input neuron signal, wij is the synaptic weight (or strength of connection) between the neurons i and j, and φ is the activation function.
While this model has seen success in machine-learning applications, it
is a poor model for real (biological) neurons, because it lacks the
time-dependence that real neuron spikes exhibit. Biological models of
the "integrate-and-fire" type take essentially this form; but they have
largely been superseded by kinetic models such as the Hodgkin–Huxley model.
In the case of modelling a biological neuron, physical analogues
are used in place of abstractions such as "weight" and "transfer
function". A neuron is filled and surrounded with water containing ions,
which carry electric charge. The neuron is bound by an insulating cell
membrane and can maintain a concentration of charged ions on either side
that determines a capacitance Cm. The firing of a neuron involves the movement of ions into the cell that occurs when neurotransmitters cause ion channels on the cell membrane to open. We describe this by a physical time-dependent current I(t). With this comes a change in voltage,
or the electrical potential energy difference between the cell and its
surroundings, which is observed to sometimes result in a voltage spike called an action potential
which travels the length of the cell and triggers the release of
further neurotransmitters. The voltage, then, is the quantity of
interest and is given by Vm(t).
Conjectures regarding the role of the neuron in the wider context of the brain principle of operation
The neurotransmitter based energy detection scheme
The neurotransmitter based energy detection scheme suggests that the neural tissue chemically executes a Radar-like detection procedure.
Fig. 6 The biological neural detection scheme as suggested by Nossenson et al
As shown in Fig. 6, the key idea of the conjecture is to account
neurotransmitter concentration, neurotransmitter generation and
neurotransmitter removal rates as the important quantities in executing
the detection task, while referring to the measured electrical
potentials as a side effect that only in certain conditions coincide
with the functional purpose of each step. The detection scheme is
similar to a radar like "energy detection" because it includes signal
squaring, temporal summation and a threshold switch mechanism,
just like the energy detector, but it also includes a unit that
emphasizes stimulus edges and a variable memory length (variable
memory). According to this conjecture, the physiological equivalent of
the energy test statistics is neurotransmitter concentration, and the
firing rate corresponds to neurotransmitter current. The advantage of
this interpretation is that it leads to a unit consistent explanation
which allows to bridge between electrophysiological measurements,
biochemical measurements and psychophysical results.
The evidence reviewed in suggest the following association between functionality to histological classification:
- Stimulus squaring is likely to be performed by receptor cells.
-
- Stimulus edge emphasizing and signal transduction is performed by neurons.
- Temporal accumulation of neurotransmitters is performed by glia cells. Short term neurotransmitter accumulation is likely to occur also in some types of neurons.
- Logical switching is executed by glia cells, and it results from exceeding a threshold level of neurotransmitter concentration. This threshold crossing is also accompanied by a change in neurotransmitter leak rate.
- Physical all-or-non movement switching is due to muscle cells and results from exceeding a certain neurotransmitter concentration threshold on muscle surroundings.
Note that although the electrophysiological signals in Fig.6 are
often similar to the functional signal (signal power / neurotransmitter
concentration / muscle force), there are some stages in which the
electrical observation is different from the functional purpose of the
corresponding step. In particular, Nossenson et al suggested that glia
threshold crossing has a completely different functional operation
compared to the radiated electrophysiological signal, and that the
latter might only be a side effect of glia break.
General comments regarding the modern perspective of scientific and engineering models
- The models above are still idealizations. Corrections must be made for the increased membrane surface area given by numerous dendritic spines, temperatures significantly hotter than room-temperature experimental data, and nonuniformity in the cell's internal structure. Certain observed effects do not fit into some of these models. For instance, the temperature cycling (with minimal net temperature increase) of the cell membrane during action potential propagation not compatible with models which rely on modeling the membrane as a resistance which must dissipate energy when current flows through it. The transient thickening of the cell membrane during action potential propagation is also not predicted by these models, nor is the changing capacitance and voltage spike that results from this thickening incorporated into these models. The action of some anesthetics such as inert gases is problematic for these models as well. New models, such as the soliton model attempt to explain these phenomena, but are less developed than older models and have yet to be widely applied.
- Modern views regarding of the role of the scientific model suggest that "All models are wrong but some are useful" (Box and Draper, 1987, Gribbin, 2009; Paninski et al., 2009).
- Recent conjecture suggests that each neuron might function as a collection of independent threshold units. It is suggested that a neuron could be anisotropically activated following the origin of its arriving signals to the membrane, via its dendritic trees. The spike waveform was also proposed to be dependent on the origin of the stimulus.

















![{\displaystyle {\frac {dV}{dt}}={\frac {1}{\tau _{m}}}[E_{m}-V+\Delta _{T}\exp \left({\frac {V-V_{T}}{\Delta _{T}}}\right)]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/adb972cf7490ca44c13d80b6b306a4a7f7a40d67)
















![{\displaystyle [t,t+\Delta _{t}]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7a5f74c8e9f1b771d10e66322f05d9692676551b)
![{\displaystyle g[s(t)]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/79f02d6c931d32be9a417598bb7164a38b18e7c7)

![{\displaystyle P_{spike}(t\in [t',t'+\Delta _{t}])=\Delta _{t}\cdot g[s(t)]}](https://wikimedia.org/api/rest_v1/media/math/render/svg/20e225ceaf3666cc8be1af1e0764ffe48fbe0475)
![{\displaystyle g[s(t)]\propto s^{2}(t)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/279ad33854b65b37667308913d562014bdb7f30a)


![{\displaystyle R_{fire}(t)={\frac {P_{spike}(t;\Delta _{t})}{\Delta _{t}}}=[y(t)+R_{0}]\cdot P_{0}(t)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d32a68d5cc75b6acd50495998a27b695e17d803a)
![{\displaystyle {\dot {P}}_{0}=-[y(t)+R_{0}+R_{1}]\cdot P_{0}(t)+R_{1}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/830fe4d93362af37a7b4d97c1902b7db1826f9e1)


![I_{{\mathrm {AMPA}}}(t,V)={\bar {g}}_{{\mathrm {AMPA}}}\cdot [O]\cdot (V(t)-E_{{\mathrm {AMPA}}})](https://wikimedia.org/api/rest_v1/media/math/render/svg/a00bcdac49e857cbf0e2440b47d7760a17d7bdc5)
![I_{{\mathrm {NMDA}}}(t,V)={\bar {g}}_{{\mathrm {NMDA}}}\cdot B(V)\cdot [O]\cdot (V(t)-E_{{\mathrm {NMDA}}})](https://wikimedia.org/api/rest_v1/media/math/render/svg/4d0bedfd5fbcaada1bfb385795d52950e6429e10)
![I_{{\mathrm {GABA_{A}}}}(t,V)={\bar {g}}_{{\mathrm {GABA_{A}}}}\cdot ([O_{1}]+[O_{2}])\cdot (V(t)-E_{{\mathrm {Cl}}})](https://wikimedia.org/api/rest_v1/media/math/render/svg/a0a4898469d8c0f8a2a1b4fd1620c3f69795f85b)
![I_{{\mathrm {GABA_{B}}}}(t,V)={\bar {g}}_{{\mathrm {GABA_{B}}}}\cdot {\tfrac {[G]^{n}}{[G]^{n}+K_{{\mathrm {d}}}}}\cdot (V(t)-E_{{\mathrm {K}}})](https://wikimedia.org/api/rest_v1/media/math/render/svg/09fdce1c61e28bac586e48a928523772a0f4187f)