A common air filter, being cleaned with a vacuum cleaner
Indoor air quality (IAQ) is the air quality within and around buildings and structures. IAQ is known to affect the health, comfort and well-being of building occupants. Poor indoor air quality has been linked to Sick Building Syndrome, reduced productivity and impaired learning in schools.
IAQ can be affected by gases (including carbon monoxide, radon, volatile organic compounds), particulates, microbial contaminants (mold, bacteria), or any mass or energy stressor that can induce adverse health conditions. Source control, filtration and the use of ventilation
to dilute contaminants are the primary methods for improving indoor air
quality in most buildings. Residential units can further improve indoor
air quality by routine cleaning of carpets and area rugs.
Determination of IAQ involves the collection of air samples,
monitoring human exposure to pollutants, collection of samples on
building surfaces, and computer modelling of air flow inside buildings.
IAQ is part of indoor environmental quality
(IEQ), which includes IAQ as well as other physical and psychological
aspects of life indoors (e.g., lighting, visual quality, acoustics, and
thermal comfort).
Indoor air pollution in developing nations is a major health hazard. A major source of indoor air pollution in developing countries is the burning of biomass (e.g. wood, charcoal, dung, or crop residue) for heating and cooking. The resulting exposure to high levels of particulate matter resulted in between 1.5 million and 2 million deaths in 2000.
Common pollutants
Second-hand smoke
Second-hand smoke is tobacco smoke which affects people other than
the 'active' smoker. Second-hand tobacco smoke includes both a gaseous
and a particulate phase, with particular hazards arising from levels of
carbon monoxide (as indicated below) and very small particulates (fine
particular matter at especially PM2.5 size, and PM10) which get into the
bronchioles and alveoles in the lung. The only certain method to improve indoor air quality as regards second-hand smoke is to eliminate smoking indoors.
Radon
Radon is an invisible, radioactive atomic gas that results from the radioactive decay of radium,
which may be found in rock formations beneath buildings or in certain
building materials themselves. Radon is probably the most pervasive
serious hazard for indoor air in the United States and Europe, and is
probably responsible for tens of thousands of deaths from lung cancer
each year.
There are relatively simple test kits for do-it-yourself radon gas
testing, but if a home is for sale the testing must be done by a
licensed person in some U.S. states. Radon gas enters buildings as a soil gas
and is a heavy gas and thus will tend to accumulate at the lowest
level. Radon may also be introduced into a building through drinking
water particularly from bathroom showers. Building materials can be a
rare source of radon, but little testing is carried out for stone, rock
or tile products brought into building sites; radon accumulation is
greatest for well insulated homes.
The half life for radon is 3.8 days, indicating that once the source is
removed, the hazard will be greatly reduced within a few weeks. Radon mitigation methods include sealing concrete slab floors, basement foundations, water drainage systems, or by increasing ventilation. They are usually cost effective and can greatly reduce or even eliminate the contamination and the associated health risks.
Radon is measured in picocuries per liter of air (pCi/L), a
measurement of radioactivity. In the United States, the average indoor
radon level is about 1.3 pCi/L. The average outdoor level is about 0.4
pCi/L. The U.S. Surgeon General and EPA recommend fixing homes with
radon levels at or above 4 pCi/L. EPA also recommends that people think
about fixing their homes for radon levels between 2 pCi/L and 4 pCi/L.
Molds and other allergens
These biological chemicals can arise from a host of means, but there
are two common classes: (a) moisture induced growth of mold colonies and
(b) natural substances released into the air such as animal dander and
plant pollen. Mold is always associated with moisture,
and its growth can be inhibited by keeping humidity levels below 50%.
Moisture buildup inside buildings may arise from water penetrating
compromised areas of the building envelope or skin, from plumbing leaks,
from condensation
due to improper ventilation, or from ground moisture penetrating a
building part. Even something as simple as drying clothes indoors on radiators can increase the risk of exposure to (amongst other things) Aspergillus
- a highly dangerous mould that can be fatal for asthma sufferers and
the elderly. In areas where cellulosic materials (paper and wood,
including drywall) become moist and fail to dry within 48 hours, mold
mildew can propagate and release allergenic spores into the air.
In many cases, if materials have failed to dry out several days
after the suspected water event, mold growth is suspected within wall
cavities even if it is not immediately visible. Through a mold
investigation, which may include destructive inspection, one should be
able to determine the presence or absence of mold. In a situation where
there is visible mold and the indoor air quality may have been
compromised, mold remediation may be needed. Mold testing and
inspections should be carried out by an independent investigator to
avoid any conflict of interest and to insure accurate results; free mold
testing offered by remediation companies is not recommended.
There are some varieties of mold that contain toxic compounds
(mycotoxins). However, exposure to hazardous levels of mycotoxin via
inhalation is not possible in most cases, as toxins are produced by the
fungal body and are not at significant levels in the released spores.
The primary hazard of mold growth, as it relates to indoor air quality,
comes from the allergenic properties of the spore cell wall. More
serious than most allergenic properties is the ability of mold to
trigger episodes in persons that already have asthma, a serious respiratory disease.
Carbon monoxide
One of the most acutely toxic indoor air contaminants is carbon monoxide
(CO), a colourless and odourless gas that is a byproduct of incomplete
combustion. Common sources of carbon monoxide are tobacco smoke, space
heaters using fossil fuels,
defective central heating furnaces and automobile exhaust. By depriving
the brain of oxygen, high levels of carbon monoxide can lead to nausea,
unconsciousness and death. According to the American Conference of Governmental Industrial Hygienists (ACGIH), the time-weighted average (TWA) limit for carbon monoxide (630-08-0) is 25 ppm.
Volatile organic compounds
Volatile organic compounds
(VOCs) are emitted as gases from certain solids or liquids. VOCs
include a variety of chemicals, some of which may have short- and
long-term adverse health effects. Concentrations of many VOCs are
consistently higher indoors (up to ten times higher) than outdoors.
VOCs are emitted by a wide array of products numbering in the thousands.
Examples include: paints and lacquers, paint strippers, cleaning
supplies, pesticides, building materials and furnishings, office
equipment such as copiers and printers, correction fluids and carbonless copy paper, graphics and craft materials including glues and adhesives, permanent markers, and photographic solutions.
Chlorinated drinking water releases chloroform when hot water is
used in the home. Benzene is emitted from fuel stored in attached
garages. Overheated cooking oils emit acrolein and formaldehyde. A
meta-analysis of 77 surveys of VOCs in homes in the US found the top ten
riskiest indoor air VOCs were acrolein, formaldehyde, benzene,
hexachlorobutadiene, acetaldehyde, 1,3-butadiene, benzyl chloride,
1,4-dichlorobenzene, carbon tetrachloride, acrylonitrile, and vinyl
chloride. These compounds exceeded health standards in most homes.
Organic chemicals are widely used as ingredients in household
products. Paints, varnishes, and wax all contain organic solvents, as do
many cleaning, disinfecting, cosmetic, degreasing, and hobby products.
Fuels are made up of organic chemicals. All of these products can
release organic compounds during usage, and, to some degree, when they
are stored. Testing emissions from building materials used indoors has
become increasingly common for floor coverings, paints, and many other
important indoor building materials and finishes.
Several initiatives envisage to reduce indoor air contamination
by limiting VOC emissions from products. There are regulations in France
and in Germany, and numerous voluntary ecolabels and rating systems
containing low VOC emissions criteria such as EMICODE, M1, Blue Angel and Indoor Air Comfort in Europe, as well as California Standard CDPH Section 01350
and several others in the USA. These initiatives changed the
marketplace where an increasing number of low-emitting products has
become available during the last decades.
At least 18 Microbial VOCs (MVOCs) have been characterised including 1-octen-3-ol, 3-methylfuran, 2-pentanol, 2-hexanone, 2-heptanone, 3-octanone, 3-octanol, 2-octen-1-ol, 1-octene, 2-pentanone, 2-nonanone, borneol, geosmin, 1-butanol, 3-methyl-1-butanol, 3-methyl-2-butanol, and thujopsene. The first of these compounds is called mushroom alcohol. The last four are products of Stachybotrys chartarum, which has been linked with sick building syndrome.
Legionella
Legionellosis or Legionnaire's Disease is caused by a waterborne bacterium Legionella
that grows best in slow-moving or still, warm water. The primary route
of exposure is through the creation of an aerosol effect, most commonly
from evaporative cooling towers or showerheads. A common source of
Legionella in commercial buildings is from poorly placed or maintained
evaporative cooling towers, which often release water in an aerosol
which may enter nearby ventilation intakes. Outbreaks in medical
facilities and nursing homes, where patients are immuno-suppressed and
immuno-weak, are the most commonly reported cases of Legionellosis. More
than one case has involved outdoor fountains in public attractions. The
presence of Legionella in commercial building water supplies is highly
under-reported, as healthy people require heavy exposure to acquire
infection.
Legionella testing typically involves collecting water samples
and surface swabs from evaporative cooling basins, shower heads,
faucets/taps, and other locations where warm water collects. The samples
are then cultured and colony forming units (cfu) of Legionella are
quantified as cfu/Liter.
Legionella is a parasite of protozoans such as amoeba, and thus requires conditions suitable for both organisms. The bacterium forms a biofilm
which is resistant to chemical and antimicrobial treatments, including
chlorine. Remediation for Legionella outbreaks in commercial buildings
vary, but often include very hot water flushes (160 °F; 70 °C),
sterilisation of standing water in evaporative cooling basins,
replacement of shower heads, and in some cases flushes of heavy metal
salts. Preventative measures include adjusting normal hot water levels
to allow for 120 °F (50 °C) at the tap, evaluating facility design
layout, removing faucet aerators, and periodic testing in suspect areas.
Other bacteria
There are many bacteria
of health significance found in indoor air and on indoor surfaces. The
role of microbes in the indoor environment is increasingly studied using
modern gene-based analysis of environmental samples. Currently efforts
are under way to link microbial ecologists and indoor air scientists to
forge new methods for analysis and to better interpret the results.
Bacteria (26 2 27) Airborne microbes
"There are approximately ten times as many bacterial cells in the
human flora as there are human cells in the body, with large numbers of
bacteria on the skin and as gut flora."
A large fraction of the bacteria found in indoor air and dust are shed
from humans. Among the most important bacteria known to occur in indoor
air are Mycobacterium tuberculosis, Staphylococcus aureus, Streptococcus pneumoniae.
Asbestos fibers
Many common building materials used before 1975 contain asbestos,
such as some floor tiles, ceiling tiles, shingles, fireproofing,
heating systems, pipe wrap, taping muds, mastics, and other insulation
materials. Normally, significant releases of asbestos fiber do not occur
unless the building materials are disturbed, such as by cutting,
sanding, drilling, or building remodelling. Removal of
asbestos-containing materials is not always optimal because the fibers
can be spread into the air during the removal process. A management
program for intact asbestos-containing materials is often recommended
instead.
When asbestos-containing material is damaged or disintegrates,
microscopic fibers are dispersed into the air. Inhalation of asbestos
fibers over long exposure times is associated with increased incidence
of lung cancer, in particular the specific form mesothelioma.
The risk of lung cancer from inhaling asbestos fibers is significantly
greater to smokers, however there is no confirmed connection to damage
caused by asbestosis . The symptoms of the disease do not usually
appear until about 20 to 30 years after the first exposure to asbestos.
Asbestos
is found in older homes and buildings, but occurs most commonly in
schools, hospitals and industrial settings. Although all asbestos is
hazardous, products that are friable, eg. sprayed coatings and
insulation, pose a significantly higher hazard as they are more likely
to release fibers to the air. The US Federal Government and some states
have set standards for acceptable levels of asbestos fibers in indoor
air. There are particularly stringent regulations applicable to schools.
Carbon dioxide
Carbon dioxide (CO2)
is a relatively easy to measure surrogate for indoor pollutants emitted
by humans, and correlates with human metabolic activity. Carbon dioxide
at levels that are unusually high indoors may cause occupants to grow
drowsy, to get headaches, or to function at lower activity levels.
Outdoor CO2 levels are usually 350-450 ppm whereas the maximum indoor CO2 level considered acceptable is 1000 ppm. Humans are the main indoor source of carbon dioxide in most buildings. Indoor CO2 levels are an indicator of the adequacy of outdoor air ventilation relative to indoor occupant density and metabolic activity.
To eliminate most complaints, the total indoor CO2 level should be reduced to a difference of less than 600 ppm above outdoor levels. The National Institute for Occupational Safety and Health (NIOSH) considers that indoor air concentrations of carbon dioxide that exceed 1,000 ppm are a marker suggesting inadequate ventilation.
The UK standards for schools say that carbon dioxide in all teaching
and learning spaces, when measured at seated head height and averaged
over the whole day should not exceed 1,500 ppm. The whole day refers to
normal school hours (i.e. 9:00am to 3:30pm) and includes unoccupied
periods such as lunch breaks. In Hong Kong, the EPD established indoor
air quality objectives for office buildings and public places in which a
carbon dioxide level below 1,000 ppm is considered to be good. European standards limit carbon dioxide to 3,500 ppm. OSHA
limits carbon dioxide concentration in the workplace to 5,000 ppm for
prolonged periods, and 35,000 ppm for 15 minutes. These higher limits
are concerned with avoiding loss of consciousness (fainting), and do not
address impaired cognitive performance and energy, which begin to occur
at lower concentrations of carbon dioxide. Given the well established
roles of oxygen sensing pathways in cancer and the acidosis independent
role of carbon dioxide in modulating immune and inflammation linking
pathways, it has been suggested that the effects of long-term indoor
inspired elevated carbon dioxide levels on the modulation of
carcinogenesis be investigated.
Carbon dioxide concentrations increase as a result of human
occupancy, but lag in time behind cumulative occupancy and intake of
fresh air. The lower the air exchange rate, the slower the buildup of
carbon dioxide to quasi "steady state" concentrations on which the NIOSH
and UK guidance are based. Therefore, measurements of carbon dioxide
for purposes of assessing the adequacy of ventilation need to be made
after an extended period of steady occupancy and ventilation - in
schools at least 2 hours, and in offices at least 3 hours - for
concentrations to be a reasonable indicator of ventilation adequacy.
Portable instruments used to measure carbon dioxide should be calibrated
frequently, and outdoor measurements used for calculations should be
made close in time to indoor measurements. Corrections for temperature
effects on measurements made outdoors may also be necessary.
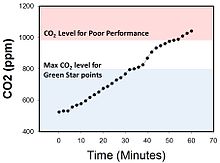
CO2 levels in an enclosed office room can increase to over 1,000 ppm within 45 minutes.
Carbon dioxide concentrations in closed or confined rooms can
increase to 1,000 ppm within 45 minutes of enclosure. For example, in a
3.5-by-4-metre (11 ft × 13 ft) sized office, atmospheric carbon dioxide
increased from 500 ppm to over 1,000 ppm within 45 minutes of
ventilation cessation and closure of windows and doors
Ozone
Ozone is produced by ultraviolet light from the Sun hitting the Earth's atmosphere (especially in the ozone layer), lightning, certain high-voltage electric devices (such as air ionizers), and as a by-product of other types of pollution.
Ozone exists in greater concentrations at altitudes commonly
flown by passenger jets. Reactions between ozone and onboard
substances, including skin oils and cosmetics, can produce toxic
chemicals as by-products. Ozone itself is also irritating to lung
tissue and harmful to human health. Larger jets have ozone filters to
reduce the cabin concentration to safer and more comfortable levels.
Outdoor air used for ventilation may have sufficient ozone to
react with common indoor pollutants as well as skin oils and other
common indoor air chemicals or surfaces. Particular concern is warranted
when using "green" cleaning products based on citrus or terpene
extracts, because these chemicals react very quickly with ozone to form
toxic and irritating chemicals as well as fine and ultrafine particles. Ventilation with outdoor air containing elevated ozone concentrations may complicate remediation attempts.
Ozone is on the list of six criteria air pollutant list. The Clean Air Act of 1990 required the United States Environmental Protection Agency to set National Ambient Air Quality Standards (NAAQS) for six common indoor air pollutants harmful to human health. There are also multiple other organizations that have put forth air standards such as Occupational Safety and Health Administration (OSHA), National Institute for Occupational Safety and Health (NIOSH), and the World Health Organization (WHO). The OSHA standard for Ozone concentration within a space is 0.1 ppm. While the NAAQS and the EPA standard for ozone concentration is limited to 0.07 ppm. The type of ozone being regulated is ground-level ozone that is within the breathing range of most building occupants
Particulates
Atmospheric particulate matter, also known as particulates, can be found indoors and can affect the health
of occupants. Authorities have established standards for the maximum
concentration of particulates to ensure indoor air quality.
Prompt cognitive deficits
In
2015, experimental studies reported the detection of significant
episodic (situational) cognitive impairment from impurities in the air
breathed by test subjects who were not informed about changes in the air
quality. Researchers at the Harvard University and SUNY Upstate Medical
University and Syracuse University measured the cognitive performance
of 24 participants in three different controlled laboratory atmospheres
that simulated those found in "conventional" and "green" buildings, as
well as green buildings with enhanced ventilation. Performance was
evaluated objectively using the widely used Strategic Management
Simulation software simulation tool, which is a well-validated
assessment test for executive decision-making in an unconstrained
situation allowing initiative and improvisation. Significant deficits
were observed in the performance scores achieved in increasing
concentrations of either volatile organic compounds (VOCs) or carbon dioxide,
while keeping other factors constant. The highest impurity levels
reached are not uncommon in some classroom or office environments.
Effect of indoor plants
Spider plants (Chlorophytum comosum) absorb some airborne contaminants
Houseplants together with the medium in which they are grown can reduce components of indoor air pollution, particularly volatile organic compounds (VOC) such as benzene, toluene, and xylene. Plants remove CO2
and release oxygen and water, although the quantitative impact for
house plants is small. Most of the effect is attributed to the growing
medium alone, but even this effect has finite limits associated with the
type and quantity of medium and the flow of air through the medium.
The effect of house plants on VOC concentrations was investigated in
one study, done in a static chamber, by NASA for possible use in space
colonies.
The results showed that the removal of the challenge chemicals was
roughly equivalent to that provided by the ventilation that occurred in a
very energy efficient dwelling with a very low ventilation rate, an air
exchange rate of about 1/10 per hour. Therefore, air leakage in most
homes, and in non-residential buildings too, will generally remove the
chemicals faster than the researchers reported for the plants tested by
NASA. The most effective household plants reportedly included aloe vera, English ivy, and Boston fern for removing chemicals and biological compounds.
Plants also appear to reduce airborne microbes and molds, and to increase humidity. However, the increased humidity can itself lead to increased levels of mold and even VOCs.
When carbon dioxide concentrations are elevated indoors relative
to outdoor concentrations, it is only an indicator that ventilation is
inadequate to remove metabolic products associated with human occupancy.
Plants require carbon dioxide to grow and release oxygen when they
consume carbon dioxide. A study published in the journal Environmental Science & Technology considered uptake rates of ketones and aldehydes by the peace lily (Spathiphyllum clevelandii) and golden pothos (Epipremnum aureum)
Akira Tani and C. Nicholas Hewitt found "Longer-term fumigation results
revealed that the total uptake amounts were 30−100 times as much as the
amounts dissolved in the leaf, suggesting that volatile organic carbons
are metabolized in the leaf and/or translocated through the petiole."
It is worth noting the researchers sealed the plants in Teflon bags.
"No VOC loss was detected from the bag when the plants were absent.
However, when the plants were in the bag, the levels of aldehydes and
ketones both decreased slowly but continuously, indicating removal by
the plants."
Studies done in sealed bags do not faithfully reproduce the conditions
in the indoor environments of interest. Dynamic conditions with outdoor
air ventilation and the processes related to the surfaces of the
building itself and its contents as well as the occupants need to be
studied.
While results do indicate house plants may be effective at
removing some VOCs from air supplies, a review of studies between 1989
and 2006 on the performance of houseplants as air cleaners, presented at
the Healthy Buildings 2009 conference in Syracuse, New York, concluded
"...indoor plants have little, if any, benefit for removing indoor air
of VOC in residential and commercial buildings."
This conclusion was based on a trial involving an unknown quantity of
indoor plants kept in an uncontrolled ventilated air environment of an
arbitrary office building in Arlington, Virginia.
Since extremely high humidity is associated with increased mold
growth, allergic responses, and respiratory responses, the presence of
additional moisture from houseplants may not be desirable in all indoor
settings if watering is done inappropriately.
HVAC design
Environmentally sustainable design
concepts also include aspects related to the commercial and residential
heating, ventilation and air-conditioning (HVAC) industry. Among
several considerations, one of the topics attended to is the issue of
indoor air quality throughout the design and construction stages of a
building's life.
One technique to reduce energy consumption while maintaining adequate air quality, is demand-controlled ventilation.
Instead of setting throughput at a fixed air replacement rate, carbon
dioxide sensors are used to control the rate dynamically, based on the
emissions of actual building occupants.
For the past several years, there have been many debates among
indoor air quality specialists about the proper definition of indoor air
quality and specifically what constitutes "acceptable" indoor air
quality.
One way of quantitatively ensuring the health of indoor air is by
the frequency of effective turnover of interior air by replacement with
outside air. In the UK, for example, classrooms are required to have
2.5 outdoor air changes per hour. In halls, gym, dining, and physiotherapy spaces, the ventilation should be sufficient to limit carbon dioxide
to 1,500 ppm. In the USA, and according to ASHRAE Standards,
ventilation in classrooms is based on the amount of outdoor air per
occupant plus the amount of outdoor air per unit of floor area, not air
changes per hour. Since carbon dioxide indoors comes from occupants and
outdoor air, the adequacy of ventilation per occupant is indicated by
the concentration indoors minus the concentration outdoors. The value of
615 ppm above the outdoor concentration indicates approximately 15
cubic feet per minute of outdoor air per adult occupant doing sedentary
office work where outdoor air contains 385 ppm, the current global
average atmospheric CO2 concentration. In classrooms, the
requirements in the ASHRAE standard 62.1, Ventilation for Acceptable
Indoor Air Quality, would typically result in about 3 air changes per
hour, depending on the occupant density. Of course the occupants are not
the only source of pollutants, so outdoor air ventilation may need to
be higher when unusual or strong sources of pollution exist indoors.
When outdoor air is polluted, then bringing in more outdoor air can
actually worsen the overall quality of the indoor air and exacerbate
some occupant symptoms related to outdoor air pollution. Generally,
outdoor country air is better than indoor city air. Exhaust gas leakages
can occur from furnace metal exhaust pipes that lead to the chimney
when there are leaks in the pipe and the pipe gas flow area diameter has
been reduced.
The use of air filters
can trap some of the air pollutants. The Department of Energy's Energy
Efficiency and Renewable Energy section suggests that "[Air] Filtration
should have a Minimum Efficiency Reporting Value (MERV) of 13 as determined by ASHRAE 52.2-1999."
Air filters are used to reduce the amount of dust that reaches the wet
coils. Dust can serve as food to grow molds on the wet coils and ducts
and can reduce the efficiency of the coils.
Moisture management and humidity control requires operating HVAC
systems as designed. Moisture management and humidity control may
conflict with efforts to try to optimize the operation to conserve
energy. For example, moisture management and humidity control requires
systems to be set to supply make-up air at lower temperatures (design
levels), instead of the higher temperatures sometimes used to conserve
energy in cooling-dominated climate conditions. However, for most of the
US and many parts of Europe and Japan, during the majority of hours of
the year, outdoor air temperatures are cool enough that the air does not
need further cooling to provide thermal comfort indoors. However, high
humidity outdoors creates the need for careful attention to humidity
levels indoors. High humidities give rise to mold growth and moisture
indoors is associated with a higher prevalence of occupant respiratory
problems.
The "dew point temperature" is an absolute measure of the
moisture in air. Some facilities are being designed with the design dew
points in the lower 50s °F, and some in the upper and lower 40s °F. Some
facilities are being designed using desiccant wheels with gas-fired
heaters to dry out the wheel enough to get the required dew points. On
those systems, after the moisture is removed from the make-up air, a
cooling coil is used to lower the temperature to the desired level.
Commercial buildings, and sometimes residential, are often kept
under slightly positive air pressure relative to the outdoors to reduce infiltration. Limiting infiltration helps with moisture management and humidity control.
Dilution of indoor pollutants with outdoor air is effective to
the extent that outdoor air is free of harmful pollutants. Ozone in
outdoor air occurs indoors at reduced concentrations because ozone is
highly reactive with many chemicals found indoors. The products of the
reactions between ozone and many common indoor pollutants include
organic compounds that may be more odorous, irritating, or toxic than
those from which they are formed. These products of ozone chemistry
include formaldehyde, higher molecular weight aldehydes, acidic
aerosols, and fine and ultrafine particles, among others. The higher the
outdoor ventilation rate, the higher the indoor ozone concentration and
the more likely the reactions will occur, but even at low levels, the
reactions will take place. This suggests that ozone should be removed
from ventilation air, especially in areas where outdoor ozone levels are
frequently high. Recent research has shown that mortality and morbidity
increase in the general population during periods of higher outdoor
ozone and that the threshold for this effect is around 20 parts per
billion (ppb).
Building ecology
It
is common to assume that buildings are simply inanimate physical
entities, relatively stable over time. This implies that there is little
interaction between the triad of the building, what is in it (occupants
and contents), and what is around it (the larger environment). We
commonly see the overwhelming majority of the mass of material in a
building as relatively unchanged physical material over time. In fact,
the true nature of buildings can be viewed as the result of a complex
set of dynamic interactions among their physical, chemical, and
biological dimensions. Buildings can be described and understood as
complex systems. Research applying the approaches ecologists use to the
understanding of ecosystems can help increase our understanding.
“Building ecology “ is proposed here as the application of those
approaches to the built environment considering the dynamic system of
buildings, their occupants, and the larger environment.
Buildings constantly evolve as a result of the changes in the
environment around them as well as the occupants, materials, and
activities within them. The various surfaces and the air inside a
building are constantly interacting, and this interaction results in
changes in each. For example, we may see a window as changing slightly
over time as it becomes dirty, then is cleaned, accumulates dirt again,
is cleaned again, and so on through its life. In fact, the “dirt” we see
may be evolving as a result of the interactions among the moisture,
chemicals, and biological materials found there.
Buildings are designed or intended to respond actively to some of
these changes in and around them with heating, cooling, ventilating,
air cleaning or illuminating systems. We clean, sanitize, and maintain
surfaces to enhance their appearance, performance, or longevity. In
other cases, such changes subtly or even dramatically alter buildings in
ways that may be important to their own integrity or their impact on
building occupants through the evolution of the physical, chemical, and
biological processes that define them at any time. We may find it useful
to combine the tools of the physical sciences with those of the
biological sciences and, especially, some of the approaches used by
scientists studying ecosystems, in order to gain an enhanced
understanding of the environments in which we spend the majority of our
time, our buildings.
Building ecology was first described by Hal Levin in an article in the April 1981 issue of Progressive Architecture magazine.
Institutional programs
The topic of IAQ has become popular due to the greater awareness of health problems caused by mold and triggers to asthma and allergies. In the US, awareness has also been increased by the involvement of the United States Environmental Protection Agency,
who have developed an "IAQ Tools for Schools" program to help improve
the indoor environmental conditions in educational institutions (see
external link below). The National Institute for Occupational Safety and Health
conducts Health Hazard Evaluations (HHEs) in workplaces at the request
of employees, authorised representative of employees, or employers, to
determine whether any substance normally found in the place of
employment has potentially toxic effects, including indoor air quality.
A variety of scientists work in the field of indoor air quality
including chemists, physicists, mechanical engineers, biologists,
bacteriologists and computer scientists. Some of these professionals
are certified by organisations such as the American Industrial Hygiene
Association, the American Indoor Air Quality Council and the Indoor
Environmental Air Quality Council.
On the international level, the International Society of Indoor
Air Quality and Climate (ISIAQ), formed in 1991, organises two major
conferences, the Indoor Air and the Healthy Buildings series. ISIAQ's journal Indoor Air
is published 6 times a year and contains peer-reviewed scientific
papers with an emphasis on interdisciplinary studies including exposure
measurements, modeling, and health outcomes.