Illustration
showing age map and zonation pattern of a monazite grain. Brighter
colour represents older age. Edited after Williams, 1999.
Monazite geochronology is a dating technique to study geological history using the mineral monazite. It is a powerful tool in studying the complex history of metamorphic rocks particularly, as well as igneous, sedimentary and hydrothermal rocks. The dating uses the radioactive processes in monazite as a clock.
The uniqueness of monazite geochronology comes from the high
thermal resistance of monazite, which allows age information to be
retained during the geological history.
As monazite grows, it forms successive generations of different
compositions and ages, commonly without erasing the previous ones,
forming zonation patterns in monazite.
Because of the age zonation, dating should be done on individual zones,
rather than the whole crystal. Also, textures of monazite crystals may
represent certain type of events. Therefore, direct sampling techniques
with high spatial resolution are required, in order to study these tiny
zones individually, without damaging the textures and zonations.
The advantage of monazite geochronology is the ability to relate
monazite compositions with geological processes. Finding the ages of
compositional zones can mean finding the ages of geological processes.
Decay of U and Th to Pb
Monazite is a rare-earth-element phosphate mineral, with the chemical formula e.g. (Ce, La, Nd, Th, Y)PO4. It appears in a small amount as an accessory mineral in many igneous, metamorphic and sedimentary rocks. Monazite minerals contain significant amounts of radioactive elements Th and U, which trigger radioactive processes. These two elements are what make this mineral suitable for radiometric dating.
In the radioactive processes, the three unstable parent isotopes
decay into their respective stable daughter isotopes of Pb. Each
following a decay chain consisting of alpha and beta decays, parent isotopes 238U, 235U and 232Th, decay into a series of intermediate daughter isotopes, and finally lead to stable isotopes, 206Pb, 207Pb and 208Pb, respectively. Each decay chain has a unique half-life, which means the daughter isotopes are generated at different rates.
The decay processes can be simplified as the following equations, which omit all the intermediate daughter isotopes.
where α represents alpha particle, β− represents beta particle, λ represents decay constant and t½ represents half-live.
Monazite geochronology studies the ratio of parent isotopes to
daughter isotopes (isotopic ratio), and calculates how much time has
passed since daughter isotopes start accumulating.
Radiometric age and geological age
Radiometric age represents the time when the decay process starts. Geological age
represents the time when a geological event occurs. Manipulating the
isotopic ratios can only give us radiometric age. To obtain the
geological age, we need to know the relationship between the two. In
other words, how do geological events affect the radioactive system in
monazite? Actually, the radioactive system is like a digital 'clock',
while the geological processes can be like replacing a battery. When a
new battery is inserted, this 'clock' starts counting from 00:00. This
process is what we call the age resetting mechanism. In monazite, the
age resetting is caused by the loss of Pb.
Pb is produced continuously by the decays of U and Th since the
radioactive system (clock) starts running. The more Pb (or less U and
Th) the system contains means the longer period has been passed. If all
Pb are suddenly removed from monazite by a geological event (replacing
battery), the age become zero (00:00) again. Before thinking what exact
geological events trigger Pb loss (see section: Interpretation and
application), it is important to know the two mechanisms causing Pb loss
in monazite.
Mechanisms of Pb loss
Solid-state diffusion
| Mineral | Tc for U-Pb dating (°C) |
|---|---|
| Titanite | 600—650 |
| Rutile | 400—450 |
| Apatite | 450—500 |
| Zircon | >1000 |
| Monazite | >1000 |
Solid-state diffusion is the net movement of atoms in solid phase,
from region of higher concentration to that of lower concentration. It
is easy to imagine diffusion in liquid phase as ink spreading in water.
Solid-state diffusion of Pb is the net exchange of Pb in the solid
mineral with the external environment, which is usually a fluid. In most
of the cases, Pb is transported from the mineral to the fluid,
resulting in Pb loss and thus age resetting.
The rate of diffusion increases with temperature as atoms are
moving faster. However, as the mineral cools and the crystal structure
becomes more complete, the diffusions of parent and daughter isotopes
slows down and finally become insignificant at a certain temperature. This closure temperature (Tc)
depends on the crystal size, shape, cooling rate and diffusion
coefficient, which in turn varies for each mineral and radioactive
systems. That is, above Tc, Pb is continuously lost and the radioactive clock is keeping zero. Once the temperature falls below Tc, the system is closed and the clock starts counting.
Monazite is characterised for its high Pb retention ability even
at high temperatures for a prolonged period. The closure temperature of
monazite in U-Th-Pb system is higher than 800 °C, much higher than the
other common minerals.
Fluid-assisted dissolution-precipitation
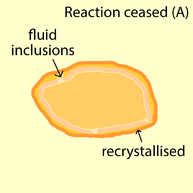
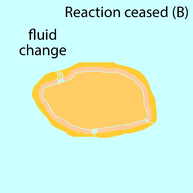
Successive growth of monazite grain by fluid-assisted dissolution-precipitation. (1) Monazite (orange) dissolves along reaction front the contact with the fluid (yellow) (2) Monazite reprecipitates as an altered monazite with a new chemical composition (pink) (3) Reaction continues with fluid being transported to the reaction front by infiltration paths. (A) Reaction ceased due to recrystallisation of precipitating phase (dark orange). (B) Reaction ceased due to change in reaction system (blue).
Unlike solid-state diffusion, fluid-assisted dissolution-precipitation occurs below Tc.
Interaction between mineral phase and coexisting fluid phase during
geological events directly contributes to this process. It is a chemical
reaction driven by the system stabilisation from minimising Gibbs free energy. A reactive fluid is present as a catalyst and a source of reactants for the reaction.
If a geological process gives a suitable fluid and temperature,
monazite dissolves along the contact with the fluid (reaction front),
and reprecipitates as an altered monazite with a new chemical
composition. The rates of the dissolution and reprecipitation are the
same, so that the original mineral phase is always in contact with the
precipitating phase, separated by only a thin layer of fluid as a
reaction medium.
Once the reaction is activated, it is self-continuing. The reaction
front migrates towards the centre of the parent monazite, leaving behind
the newly formed monazite, forming a core-rim structure.
The composition of the precipitating phase depends on the fluid
composition and temperature. During most of the reactions, Pb is
efficiently removed and the precipitating phase is Pb-free. Therefore, the age of the newly formed rim is reset, representing the time of this alternation.
There are basically two factors causing the reaction to cease. (A)
Reaction ceases due to the recrystallisation of precipitating phase,
removing all the fluid infiltration paths. This results in fluid
inclusion in monazite. (B) Reaction ceases due to change in system such as composition of fluid and monazite, making this reaction no longer reactive.
Implications for monazite geochronology
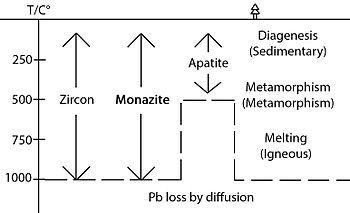
Range of geological processes at different temperature recorded by monazite, zircon and apatite in U-Pb dating
Since
the diffusion of reactants between dissolving phase and precipitating
phase is slow, the fluid is essential to provide an easy transport for
the reactants. Yet as reaction proceeds, dissolving phase and the fluid
are separated by the solid precipitating phase, blocking the transport
of reactants. Therefore, there must be some inter-connected porosity in
the precipitating phase, which allows the fluid to infiltrate and fuel
the reaction front.
Most other geochronometers usually have a much lower closure temperature. Once they are subject to a temperature higher than Tc, all age information will be reset, losing information of the past geological events. In contrast, since monazite has a high Tc, even it experiences younger high-grade metamorphism
with high temperatures, it is likely that the previous geological
history is preserved. Furthermore, dissolution-precipitation is usually
triggered by geological events such as metamorphism, deformation and hydrothermal alternation below Tc.
Each of these events writes a new age information by precipitating a
new domain without erasing the older information. Therefore, it is
likely that monazite preserves a complete history of generations.
Monazite and zircon are two minerals that commonly employed in geochronology to study geological history.
They both exhibits high closure temperatures which make them suitable
to reveal igneous and metamorphic events. However, they behave
differently throughout the geological history.
Generally, monazite performs better in recording metamorphism
(recrystallisation ages) with different zonation patterns in ages and
composition. Zircon is not as reactive as monazite during metamorphism
reaction and better in recording igneous events (cooling ages). Moreover, monazite is suitable in dating relatively low-temperature metamorphism for example amphibolite-facies than zircon.
Monazite zonation
Zonation
is a characteristic of monazite. A single monazite grain can contain
domains of distinctively different compositions and ages. These domains
are widely accepted to represent episodes in geological history during
monazite growth or recrystallisation.
The key to monazite geochronology is to find out what geological events
or environments a domain is representing, by comparing its chemical
composition with mineral stability and reactions. The age of the event
is thus represented by the domain age.
The ideal formula of monazite is [LREE(PO4)], the variation in composition is mainly due to the chemical substitutions of light rare earth elements
(REE) in monazite by other elements. One of the common substitutions
are the exchange between LREE with Th and Ca, and P with Si to form huttonite [Th(SiO4)] and brabantite [CaTh(PO4)2]. Since all three minerals share the same chemical structure, they are the three endmembers in their solid solution,
meaning that they appear in a same solid phase where substitutions
happen. It is important to note that the composition zonation pattern
may not be the same when we are considering different elements. And age
zonation may have no relationship with composition zonation at all. (see
images from the section: analysis procedures) Thus, it needs to be very
careful in linking among zonations. In natural monazite, the zonation
pattern maybe complex and hard to interpret. Below we describe some
simple chemical zonation patterns and the associated interpretations.
Zonation patterns associated with igneous activity are usually easy to
interpret. However, those associated with metamorphism are more
complicated.
Concentric zoning
One of the monazite formations is crystallization of an igneous melt.
The concentric zoning pattern reflects the changing composition of the
melt which affect the crystallising composition of monazite.
Sector zoning
Sector
zoning is also associated with the crystallization of monazite in a
melt. However, some elements may have a tendency to crystallise onto a
specific crystal face. It results in uneven growth and composition around monazite.
Core-rim zoning
Core-rim
zoning is usually associated with the fluid-assisted
dissolution-precipitation in metamorphic reactions, forming rims with
new composition. The fluid composition and metamorphic grade (H/T) are important factors on the rim composition.
Other zoning patterns
Mottled and patchy zoning patterns are the more complicated zonations. The interpretations are usually not simple.
Dating approaches
Isotopic
dating and chemical dating are the two typical dating approaches used
in monazite geochronology. Both methods make use of the radioactive
nature of Th and U in monazite.
Isotopic dating
Isotopic dating requires measuring the isotopic concentration of
radioactive U and Th, and radiogenic Pb in monazite. By treating each
decay chain in the U-Th-Pb system independently, three classic isochron
equations can be obtained:
where represents the initial isotopic ratio when the system resets, t represents the time after the system reset, and λ238, λ235 and λ232 are the decay constants of 238U, 235U and 232Th respectively.
Combinations of the use of the above equations, such as U-Th-Pb dating, U-Pb dating
and Pb-Pb dating, require different levels of analysis techniques and
offer variable levels of precision and accuracy. The general uncertainty
in the ages measured is 2σ (e.g.).
Chemical dating/ Total Pb dating
Chemical dating
requires measuring the elemental abundances of U, Th and Pb but not
isotopes. U-Th-total Pb dating, also known as electron microprobe
U–Th–Pb dating, measures the elemental abundances of the three elements
by an electron microprobe, and calculate the age (t) by the below equation.
where Pb, Th and U are concentrations in parts-per-million, and λ232, λ235 and λ238 are the decay constants of 232Th, 235U and 238U respectively.
For chemical dating results to be valid, the following assumptions are required:
- Non-radiogenic Pb is negligible compared to radiogenic Pb.
- No modification of U/Th/Pb has occurred except radioactivity.
The first assumption tends to be true since monazite is very unlikely
to incorporate Pb during its growth. The non-radiogenic Pb content in
many laboratory tests were found to be very low, nearly always lower
than 1 ppm. The most common error arose from this assumption is the contamination with lead during sample preparation.
The second assumption is usually justified by the concordant behavior
of the mineral observed in tests. That means the system is either reset
totally or unaffected totally by geological processes, there is not
partial resetting of the system. Minor errors are arose due to
negligible disturbance during mass transfer.
The theory is that monazite has high contents of Th (generally
3-15% and up to 25% of its weight) and U (generally hundreds of ppm and
up to 5% in concentration). Thus, Pb accumulates at a high rate by
radioactive processes. In less than hundreds of years, it reaches a
level high enough to be measured accurately by an electron microprobe.
Analysis techniques
Age
and composition zonations as well as the texture of monazite provide
evidence on the successive growth of the crystal during geological
events. The scope of such information that can be obtained largely
depends on the analysis techniques employed in geochronology.
Comparison between convectional and in situ analysis
Convectional analysis
Conventionally, monazite is separated from samples by dissolution
and chemical methods. Single or fractions of crystals are selected for
dating, usually by thermal ionization mass spectrometry
(TIMS). That means one age is generated for a single monazite crystal
or for a group of crystals. The age information obtained is obviously
inconsistent and inaccurate, because even a single monazite crystal
contains zones of different ages. Also, the mechanical separation for
monazite often destroy the associated textual and spatial information of
monazite, which is crucial in interpreting relationship between domains
and geological environment.
In situ analysis
| Convectional analysis | In situ analysis | |
|---|---|---|
| Sampling | Physical/chemical separation | Direct sampling |
| Dating target | Single/ fractions of grains | Age domains |
| Dated age | Inconsistent | Consistent |
| Texture preserved? | No | Yes |
For the above reasons, the demand for in situ analysis is increasing.
In situ means analyzing monazite at its original place without monazite
separation (refer to in situ)
such that the texture and zonation pattern are kept intact in order to
reveal a more comprehensive geological history of the host rock.
Direct sampling techniques, high spatial resolution and precision are
the requirements for an in situ analysis. With technological
advancement, more and more measurement tools such as laser ablation inductively coupled plasma mass spectrometry (LA-ICPMS) and laser microprobe mass spectrometer (LMMS) become capable for such analysis.
Analysis procedures
Below
shows a general procedure for monazite dating. The characteristics and
procedure are different for each measurement tool, especially sample
preparation and dating method. Details of some common measurement tools are described in the section: Measurement tools.
- Sample preparation
- Monazite identification and mapping
- Monazite compositional mapping
- Monazite age mapping
- Quantitative dating
Monazite
identification: Illustration showing backscattered electron image of a
rock sample with monazite at the centre with white colour. Edited after
Williams, 1999.
Compositional
mapping: Illustration showing X-ray Th composition map of a monazite
grain. Brighter colour represents higher concentration. Edited after
Williams, 1999.
Quantitative dating: Histogram of age measured, showing two age zonations in monazite. Edited after Williams, 1999.
Sample preparation
In both conventional and in-situ dating, a thin section of the rock in interest is prepared.
First, a thin layer of rock is cut by a diamond saw and ground to
become optically flat. Then, it is mounted on a slide made of glass or
resin, and ground smooth using abrasive grit. The final sample is
usually only 30 μm thick.
Monazite identification and mapping
Monazite grains are identified by backscattered electron imaging survey or/and electron microprobe analysis
(EMPA) by mapping concentration of distinctive Ce in monazite. The two
images are usually superimposed to reflect sample texture and monazite
locations at the same time.
Monazite compositional mapping
Monazite
grains which show useful relations with microtextures or host minerals
are selected for compositional mapping. Major elemental and sometimes
trace elemental maps are created at high magnification by electron
microprobe X-ray mapping to show composition zonation patterns. Maps of elemental Y, Th, Pb, U have been proven useful in identifying composition domains in monazite.
Monazite age mapping
Estimated
ages are calculated across the compositional map by analysing the
concentration of Th, Ph and U by total-Pb dating method. The result is then used to generate an age map which approximately identifies all the age domains.
Quantitative dating
A
number of spots within an age domains are selected and further dated
accurately with the measurement tools by isotopic dating method. The results are then analysed statistically to give an accurate age of each age domain.
Measurement techniques
Employment
of different analysis techniques (conventional or in situ analysis)
provides selection of different measurement techniques. Choice between
these techniques in turn affects the resolution, precision, detection
limits and costs of monazite geochronology. The recent analytical
progress in U-Th-Pb system in natural monazite has been mainly achieved
by (1) Isotope Dilution Thermal Ionization Mass Spectrometry (ID-TIMS), (2) Secondary Ion Mass Spectrometry (SIMS), (3) Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA-ICP-MS) and (4) Electronic Microprobe Analyses (EMPA).
Conventional analysis
Isotope dilution thermal ionization mass spectrometry
In the 1950s, Alfred Nier
developed the technique of ID-TIMS, which later become the first tool
used in monozite geochronology. Since this method involves the chemical
separation of monazite (isotope dilution), it is regarded as a
conventional analysis technique. Generally, it takes several hours for a
U-Pb measurement. The precision of date is nearly 0.1%, provided that
the ages are concordant (not dating with mixing of zonations). It is
regarded as the most precise method in monozite geochronology.
Monazite mineral grains selected are carefully hand-picked for
dating. They are spiked with a tracer solution and dissolved in HF or
HCl. Using ion exchange chemistry, U, Th and Pb are separated from other elements. The purposes of the separation are (1)
potential isobaric interference should be removed before analysis
because of the high-sensitivity and low-mass resolution nature of TIMS; (2) ionization of the interested elements maybe impeded by other elements, which results in reduced signal size and precision.
The separated U, Th and Pb samples are put carefully onto a metal filament, which is usually made from Re.
The elements are heated and ionize to the respective ions, which
accelerate under strong magnetic field and are measured by a detector.
The tracer solution is a solution with a known amount of U and Pb
tracer isotopes. Due to elemental fractionation, both elements cannot
be measured simultaneously by TIMS. The tracer solution is therefore
used to measure ratios of sample isotope to tracer isotopes. The ratios
are converted to moles of sample isotopes for dating.
In situ analysis
The
following measurement techniques applies in in situ analysis which
involves direct sampling of monazite grains using an incident ion beam
or a laser.
Secondary ion mass spectrometry
Old magnetic sector SIMS by the manufacturer CAMECA
SIMS is a mass spectrometry method to measure small-scale elemental and isotopic variations of samples.
Its ability to measure in spots with small-diameter (10-40 μm) makes it
a useful tool to date small (less than 100 μm) minerals and domains in a
single crystal for monozite geochronology. SIMS can achieve a precision
of ~3% on the dates. Sensitive high-resolution ion microprobe (SHRIMP) is widely regarded as a powerful tool among SIMS.
SIMS analyzes the mineral surface (a few μm) composition by sputtering the surface with a focused primary ion beam
under vacuum. The secondary ions liberated from the mineral are
accelerated, analysed and measured in the mass spectrometer. Sample are
analysed in rotation with standard with known elemental or isotopic
ratios in order to measure the ratios in the sample for dating.
Laser ablation inductively coupled plasma mass spectrometry
The application of LA-ICPMS in U-Pb geochronology started in the
1990s. Since it enables relatively short and cheap yet
high-spatial-resolution analysis, it has become the most utilized method
of monazite geochronology. The precision of LA-ICPMS is limited by standard variability, which is about 2% on date.
Mineral sample surface is sputtered by a laser inside a sample
cell. The ablated particles are collected and incorporated into a
carrier gas. The resulting aerosols is analyzed by a mass spectrometer
for dating. A solid-state or gas-source laser with short wavelength is
commonly used as the laser ablation system in geochronology.
Electronic microprobe analyses
EMPA is employed in monazite geochronology especially in in situ chemical dating (total-Pb dating).
The high content of U, Th and Pb in monazite match with the requirement
arose from the relatively higher lower detection limit. Therefore, EMPA
is thus a high-resolution (approximately 1 μm), rapid and inexpensive
method in chemical dating to resolve growth histories of monazite. It can achieve a precision of 5—10 myr in Pb-rich monazite, and 10—20 myr in Pb-poor monazite.
Interpretation and application
Monazite
geochronology can reveal complex geological history recorded in the
monazite mineral grains. The characteristic composition and age
zonations are the basic for carrying out such analysis, with each domain
representing a past geological event with a certain age. The most
important issue in monazite geochronology is to relate textures and
compositions in each domain to the associated geological events.
Even for a single monazite grain may reveal complex history, in
which events maybe inter-related or even happen at the same time, making
it hard to clearly separate each event for discussion. The below
section aims to provide briefly how composition and age data are
interpreted to link different types of events.
Crystallisation of melt
Understanding the igneous petrology of monazite is important to date crystallisation age of igneous rocks. Monazite is commonly present as accessory mineral in low-CaO peraluminous granitoids, from diorites, micaceous granites to pegmatites. The reason of the low CaO content is probably that melts with high CaO content promotes the formation of apatite and allanite but not monazite. It is commonly formed from the magmatism involving carbonatic melts but not mafic plutons or lavas. Those rocks usually host economic REE ore deposits, making monazite geochronology important in mining exploration.
The simplest monazite zonation showing successive crystallisation
of melts is concentric zonation, with new monazite crystallised as rims
by rims surrounding the core. The rims often shows compositional
variation due to the preferential incorporation of certain elements in
the crystal lattice. For example, considering a closed system, Th is
preferentially incorporated into the monazite mineral structure, leaving
Th-depleted melt. Therefore, older monazite is rich in Th while younger
monazite contains less Th. This results in a rimward decrease of Th in a
concentric zoning pattern. Investigating composition and age variation
of these rims help to constrain the timing and rate of crystallisation
as well as the composition of the melt, especially for rocks where
zircon is not present for zircon dating.
Monazite – cheralite – huttonite system
Monazite geochronology can also reveal igneous differentiation events such as magma mixing, where the magma chamber
is evolved into different composition. Isomorphous substitution is one
of the examples. It is a form of substitution where one element is
replaced by another without changing the crystal structure. In the case
of monazite, the rare earth elements are replaced by Ca and Th.
Different level of substitution form a range of compositions, with endmembers monazite [2REE(PO4)], brabantite [Ca,Th(PO4)2] and huttonite [2ThSiO4]. The level of substitution usually depends composition of melt and thus the geological environment.
Hydrothermal alternation
Illustration showing clusters formed by multiple crystals. Edited after Schandl (2004)
Hydrothermal process is usually coupled with igneous process.
Monazite geochronology helps studying the development from igneous
process to Hydrothermal process, and revealing later hydrothermal alternation, which is vital in the study of ore formation.
Although it is hard to distinguish between magmatic monazite and
hydrothermal monazite, analysing the texture and the occurrence pattern
of monazite may help distinguishing them.
Hydrothermal monazites tend to appear in clusters formed by multiple
crystals, while igneous monazites tend to appear homogenous throughout
the rock. Also, hydrothermal monazites usually contain low ThO2 content. These distinctive features can be easily identified with textual and compositional analysis in monazite geochronology.
Metamorphism
Monazite geochronology is generally regarded as a powerful tool to reveal metamorphic history. Metamorphism
is the mineral change of textural change in preexisting rocks in
response to a change in environment with different temperatures and
pressures. It occurs at a temperature above diagenesis (~200 °C) and below melting (>800 °C). The mineral assemblage formed under metamorphism depends on the composition of the parent rock (protolith)
and more importantly, the stability of different minerals in varying
temperature and pressure (P-T). A set of mineral assemblage that formed
under similar temperature and pressure is called metamorphic facies.
Actually, most mineral changes during rock burial, uplift, hydrothermal
processes and deformation are associated with metamorphic reactions.
Monazite is commonly found in many metamorphic rocks, especially in those formed from pelites and sandstones.
The zonation in monazite reflects the successive monazite forming
events. They may be formed from reactions along a single
pressure-temperature (P-T) loop in a phase diagram, or reactions without changing P-T. For a metamorphic event, monazite is formed by the reactions with more than one P-T loop.
The objective of monazite geochronology is to relate these
monazite forming events/reactions with P-T conditions. We can then put
time constrains on the P-T loops, forming a comprehensive
pressure-temperature-time loops revealing the metamorphic history of the
rocks.
Monazite inclusions in metamorphic porphyroblasts and matrix
Different porphyroblasts like garnet and quartz are often formed during metamorphism in different ranges of P-T. Monazite grains are often found as inclusion
in porphyroblasts. Since the host mineral monazite is quite thermally
resistant, these inclusions are protected from age resetting, even at a
prolonged exposure at temperature higher than 800 °C, this enables us to restrict an upper limit of the age of the porphyroblasts, and thus the associated metamorphic events.
For example, a metamorphic rock in the Neil Bay area of northern Saskatchewan underwent high grade (high P/T) metamorphism followed by exhumation (uplift). Porphyroblast garnet is formed during high grade metamorphism while porphyroblast cordierite
is formed during exhumation afterwards. Both porphyroblasts contain
monazite inclusions which dated 1910 Ma and 1840 Ma respectively. And matrix
monazite is dated 1800 Ma. Thus, it is interpreted that high grade
metamorphism occurred after 1910 Ma and before 1840 Ma, while exhumation
after 1840 Ma, and the final annealing (cooling and coarsening of
minerals) at 1800 Ma.
Within the same setting as above, monazite inclusions in garnet
maybe either younger, older than or have similar ages with the matrix
monazite. Both of them may even have a wide range of ages with no
systematic distribution.
These scenarios are interpreted to represent different metamorphic
paths and conditions, giving varying or complex sequences of metamorphic
reactions.
Elemental fractionation between monazite and silicates
Elemental
fractionation refers to the difference between the amount of element
incorporated into the solid mineral phase and the amount of element
stayed in the liquid fluid phase. Minerals have the characteristic of
preferential intake of certain elements during its growth. For example,
as monazite grows in size, it preferentially incorporate Th in the
crystal structure. It results in less available Th in the environment
for future growth. Thus, younger monazite tends to have lower Th
contents. It provides one of the reasons for the compositional variation of monazite.
When considering the whole system of metamorphic rocks, there are
also other minerals which shows elemental fractionation. The interplay
between fractionations in monazite and these minerals has a great impact
on the compositional zonation of monazite.
The interplay is often caused by the formation and breakdown of the
minerals, which is in turn a result of different stages in P-T paths. Dating fractionating zonation thus help putting time constraint on metamorphism.
P-T path corresponding to formation of low-Y core and high-Y rim of monazite
The mostly studied system is the yttrium
(Y) fractionation between monazite and silicates garnet and xenotime.
All three minerals preferentially fractionate Y, yet they form and break
down at different stage of metamorphism. Xenotime has the highest
fractionating power, then garnet and then monazite. In a simplified case
of a clockwise P-T path involving garnet and monazite, garnet grows
along prograde path with Y continuously incorporated, thus the Y content
in monazite formed at this stage (prograde) should decrease
progressively with higher grade. However, as temperature increases to a
certain point, partial melting
(anatectic) of monazite occurs and it dissolves along the rim,
releasing Y into the melts. As the system later cools and melt
crystallises, regrowing monazite will have higher Y content.
Partial melting usually happen during peak metamorphism (highest
temperature in P-T path), but age and chemical information during this
stage are not recorded since the monazite is melting. However, the ages
of last prograde growth rim (lowest Y) and the first post-anatectic
growth rim (highest Y) usually bracket the time of partial melting.
Another scenario involves the formation or breakdown of garnet, influencing the Y and HREE (heavy rare earth elements) content in the environment, thus the content of growing monazite. Basically, monazite growth before garnet formation has a higher Y and HREE content than those during or after garnet formation. As garnet start breaking down in the later stage of metamorphism, the monazite forms rims rich in Y and HREE.
The extent of fractionation of Y between garnet and monazite is
also found to be related to temperature. It is thus used as a
thermometer, providing the temperature constrain on the P-T path.
Deformation
Timing
deformation events is one of the important components in tectonic
study. Large scaled cross-cutting relationships between rocks, dikes and
plutons easily provide certain but relatively broad time constrain on
deformation. In contrast, monazite can itself be participated in
deformation fabric, reaction and fracture, thus studying microfabrics
and microtextures of monazite offers a more straightforward method of
dating a deformation event.
Deformation metamorphic reaction
Deformation
events may trigger metamorphic reactions which produce monazite. For
example, a metamorphic reaction associated with the movement in the Legs
Lake shear zone partly replaced garnet with cordierite.
This reaction also generated new monazite with high content of Y, and
dated around 1850 Ma. The age is treated as the timing of shearing.
One point to notice is those monazite forming reactions may happen a bit later than the shearing
after the rocks have been in re-equilibrium in response to a new pressure environment. That means monazite age may not be equivalent to shearing age, yet it provides a more precise age than the other methods.
Monazite deformation fabric
Monazite grain is aligned with foliation S1. New monazite overgrowth grows along S1 direction. Edited after Mccoy, 2005.
Monazite mineral itself can form fabric caused by deformation.
Monazite may be present as elongate grains aligned in foliation. It can
be interpreted that the monazite is formed before the shearing and align
during shearing, or formed at the same time of shearing.
It thus provides an upper limit of the shearing age. For example, if
the monazite is dated 800 Ma, the age of shearing cannot be older than
800 Ma.
However, it can also be interpreted that the monazite grew along
the foliation of other minerals long after the shearing. This problem
can be solved by analysing the compositional domains of monazite.
Monazite along exiting foliation would have a tendency to grow at the
two ends along the foliation.
If we can find monazite overgrowth with different composition and age
along at the two opposite ends of the grain, it is likely that date of
monazite overgrowth is younger than shearing.
Monazite fracture
Schematic
diagram showing monazite fracture and refilling monazite. The monazite
crystal with lighter colour is fractured by shearing. Later new monazite
with new composition with darker colour forms along the fracture.
Modified from Shaw (2001).
Fracture and offset in a single monazite crystal have been observed
mimicking a bookshelf fault in a large-scale fracturing event.
The fractured grain is dated 1375 Ma, indicating that the large-scale
displacement happened after this date. Moreover, new monazite may later
grow and fill up the space created by the fracture, enclosing the time
constrain completely. For example, if the new monazite is dated 1200 Ma, the displacement probably occurred within 1375—1200 Ma.
Sedimentary events
Detrital monazite
Detrital monazite is the monazite particles that produced from the
weathering and erosion of pre-existing rocks. The weathered monazite
grains are produced in the source and then transferred into sedimentary basins
by erosion. The detrital monazite contains zonation patterns which
preserve the geological history of the source region. Investigating
detrital monazite in the basin not only help constructing the
metamorphic, tectonic and hydrothermal history of the source region, but
also finding deposition age, structural evolution and sediment source
of the basin.
For example, the domain with youngest age may represent exhumation of
source rock, which is followed by immediate erosion and deposition.
Diagenetic monazite
Diagenetic monazite is the monazite that formed during or after the lithification
of sedimentary rocks. Monazite has been observed to grow on the other
minerals or in the pore spaces during diagenesis of sediments.
Studying diagenetic monazite provides a good method to study age,
geochemical and thermal evolution of sedimentary basins, in particular
those in Precambrian that with little fossil controls.
Industrial Use
U-Th-Pb data and monazite ages can be used as a valuable tool for prospecting. It was shown for 3 localities in Pisecke Hory Region, the Czech Republic.