Nickel superalloy jet engine (RB199) turbine blade
A superalloy, or high-performance alloy, is an alloy that exhibits several key characteristics: excellent mechanical strength, resistance to thermal creep deformation, good surface stability, and resistance to corrosion or oxidation. The crystal structure is typically face-centered cubic austenitic. Examples of such alloys are Hastelloy, Inconel, Waspaloy, Rene alloys, Incoloy, MP98T, TMS alloys, and CMSX single crystal alloys.
Superalloy development has relied heavily on both chemical and
process innovations. Superalloys develop high temperature strength
through solid solution strengthening. An important strengthening mechanism is precipitation strengthening
which forms secondary phase precipitates such as gamma prime and
carbides. Oxidation or corrosion resistance is provided by elements such
as aluminium and chromium.
The primary application for such alloys is in turbine engines, both aerospace and marine.
Chemical development
Because
these alloys are intended for high temperature applications (i.e.
holding their shape at temperatures near their melting point) their creep and oxidation resistance are of primary importance. Nickel (Ni) based superalloys have emerged as the material of choice for these applications.
The properties of these Ni based superalloys can be tailored to a
certain extent through the addition of many other elements, both common
and exotic, including not only metals, but also metalloids and nonmetals; chromium, iron, cobalt, molybdenum, tungsten, tantalum, aluminium, titanium, zirconium, niobium, rhenium, yttrium, vanadium, carbon, boron or hafnium
are some examples of the alloying additions used. Each of these
additions has been chosen to serve a particular purpose in optimizing
the properties for high temperature application.
Creep resistance is dependent, in part, on slowing the speed of dislocation motion within a crystal structure. In modern Ni based superalloys, the γ’-Ni3(Al,Ti) phase present acts as a barrier to dislocation motion. For this reason, this γ’ intermetallic
phase, when present in high volume fractions, drastically increases the
strength of these alloys due to its ordered nature and high coherency
with the γ matrix. The chemical additions of aluminum and titanium
promote the creation of the γ’ phase. The γ’ phase size can be
precisely controlled by careful precipitation strengthening heat
treatments. Many superalloys are produced using a two-phase heat
treatment that creates a dispersion of cuboidal γ’ particles known as
the primary phase, with a fine dispersion between these known as
secondary γ’. In order to improve the oxidation resistance of these
alloys, Al, Cr, B, and Y are added. The Al and Cr form oxide layers that
passivate the surface and protect the superalloy from further oxidation
while B and Y are used to improve the adhesion of this oxide scale to
the substrate. Cr, Fe, Co, Mo and Re all preferentially partition to the γ matrix
while Al, Ti, Nb, Ta, and V preferentially partition to the γ’
precipitates and solid solution strengthen the matrix and precipitates
respectively. In addition to solid solution strengthening, if grain
boundaries are present, certain elements are chosen for grain boundary
strengthening. B and Zr tend to segregate to the grain boundaries which
reduces the grain boundary energy and results in better grain boundary
cohesion and ductility.
Another form of grain boundary strengthening is achieved through the
addition of C and a carbide former, such as Cr, Mo, W, Nb, Ta, Ti, or
Hf, which drives precipitation of carbides at grain boundaries and
thereby reduces grain boundary sliding.
While Ni based superalloys are excellent high temperature
materials and have proven very useful, Co based superalloys potentially
possess superior hot corrosion, oxidation, and wear resistance as
compared to Ni-based superalloys. For this reason, efforts have also
been put into developing Co based superalloys over the past several
years. Despite that, traditional Co based superalloys have not found
widespread usage because they have a lower strength at high temperature
than Ni based superalloys.
The main reason for this is that they appear to lack the γ’
precipitation strengthening that is so important in the high temperature
strength of Ni-based superalloys. A 2006 report on metastable γ’-Co3(Al,W) intermetallic compound with the L12
structure suggests Co based alloys as alternative to traditional Ni
based superalloys. However this class of alloys was reported in a PhD
thesis by C. S. Lee in 1971.
The two-phase microstructure consists of cuboidal γ’ precipitates
embedded in a continuous γ matrix and is therefore morphologically
identical to the microstructure observed in Ni based superalloys. Like
in the Ni-based system, there is a high degree of coherency between the
two phases which is one of the main factors resulting in the superior
strength at high temperatures. This provides a pathway for the
development of a new class of load-bearing Co based superalloys for
application in severe environments. In these alloys 'W' is the crucial addition for getting γ’ intermetallic compound that makes them much denser (>9.6 g/cm3)
compared to Ni-based superalloys. Recently a new class of γ - γ’
cobalt based superalloys have been developed that are "W" free and have
much lower density comparable to nickel based superalloys.
In addition to the fact that many of the properties of these new Co
based superalloys could be better than those of the more traditional Ni
based ones, Co also has a higher melting temperature than Ni. Therefore,
if the high temperature strength could be improved, the development of
novel Co based superalloys could allow for an increase in jet engine
operation temperature resulting in an increased efficiency.
Metallurgy of superalloys
Ni-based superalloy phases
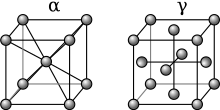
- Gamma (γ): This phase composes the matrix of Ni-based superalloy. It is a solid solution fcc austenitic phase of the alloying elements. Alloying elements found in most commercial Ni-based alloys are, C, Cr, Mo, W, Nb, Fe, Ti, Al, V, and Ta. During the formation of these materials, as the Ni-alloys are cooled from the melt, carbides begin to precipitate, at even lower temperatures γ' phase precipitates.
- Gamma Prime (γ'): This phase constitutes the precipitate used to strengthen the alloy. It is an intermetallic phase based on Ni3(Ti,Al) which have an ordered FCC L12 structure. The γ' phase is coherent with the matrix of the superalloy having a lattice parameter that varies by around 0.5%. Ni3(Ti,Al) are ordered systems with Ni atoms at the cube faces and either Al or Ti atoms at the cube edges. As particles of γ' precipitates aggregate, they decrease their energy states by aligning along the <100> directions forming cuboidal structures. This phase has a window of instability between 600 °C and 850 °C, inside of which γ' will transform into the HCP η phase. For applications at temperatures below 650 °C, the γ" phase can be utilized for strengthening.
Crystal structure for γ" (Ni3Nb) (Body Centered Tetragonal)
- Gamma Double Prime (γ"): This phase typically possesses the composition of Ni3Nb or Ni3V and is used to strengthen Ni-based superalloys at lower temperatures (<650 a="" class="mw-redirect" crystal="" href="https://en.wikipedia.org/wiki/Body-centered_tetragonal" is="" nbsp="" of="" relative="" structure="" the="" title="Body-centered tetragonal" to="">body-centered tetragonal
Co-based superalloy phases
- Gamma (γ): Similar to Ni-based superalloys, this is the phase of the superalloy’s matrix. While not used commercially to the extent of Ni-based superalloys, alloying elements found in research Co-based alloys are C, Cr, W, Ni, Ti, Al, Ir, and Ta. Chromium is also used in Cobalt based superalloys (occasionally up to 20 wt.%) as it provides oxidation and corrosion resistance, critical for material use in gas turbines.
- Gamma Prime (γ'): Just as in Ni-based super alloys, this phase constitutes the precipitate used to strengthen the alloy. In this case, it is usually close packed with a L12 structure of Co3Ti or fcc Co3Ta, though both W and Al have been found to integrate into these cuboidal precipitates quite well. The elements Ta, Nb, and Ti integrate into the γ’ phase and are quite effective at stabilizing it at high temperatures. This stabilization is quite important as the lack of stability is one of the key factors that makes Co-based superalloys weaker than their Ni-base cousins at elevated temperatures.
- Carbide Phases: As is common with carbide formation, its appearance in Co-based superalloys does provide precipitation hardening, but does decrease low-temperature ductility.
Fe-based superalloy phases
The
use of steels in superalloy applications is of interest because certain
steel alloys have showed creep and oxidation resistance similar to that
of Ni-based superalloys, while being far less expensive to produce.
Gamma (γ): Like the phases found in Ni-based superalloys,
Fe-based alloys feature a matrix phase of austenite iron (FCC). Alloying
elements that are commonly found in these stainless steel alloys
include: Al, B, C, Co, Cr, Mo, Ni, Nb, Si, Ti, W, and Y.
While Al is introduced for its oxidation benefits, Al additions must be
kept at low weight fractions (wt.%) because Al stabilizes a ferritic
(BCC) primary phase matrix, which is an undesirable phase in superalloy
microstructures, as it is inferior to the high temperature strength
exhibited by an austenitic (FCC) primary phase matrix.
Gamma-Prime (γ’): This phase is introduced as precipitates to
strengthen the alloy. Like in Ni-based alloys, γ’-Ni3Al precipitates can
be introduced with the proper balance of Al, Ni, Nb, and Ti additions.
Microstructure of Fe-based superalloys
Two
major types of austenitic stainless steels exist and are characterized
by the oxide layer that forms at the surface of the steel:
chromia-forming or alumina-forming stainless steel. Chromia-forming
stainless steel is the most common type of stainless steel produced.
However, chromia-forming steels do not exhibit high creep resistance at
high operating temperatures, especially in environments with water
vapor, when compared to Ni-based superalloys. Exposure to water vapor at
high operating temperatures can result in an increase in internal
oxidation in chromia-forming alloys and rapid formation of volatile Cr
(oxy)hydroxides, both of which can reduce the durability and lifetime of
the alloy.
Alumina-forming austenitic stainless steels feature a
single-phase matrix of austenite iron (FCC) with an alumina oxide at the
surface of the steel. Alumina is more thermodynamically stable in
oxygen than chromia. More commonly, however, precipitate phases are
introduced to increase strength and creep resistance. In alumina-forming
steels, NiAl precipitates are introduced to act as Al reservoirs to
maintain the protective alumina layer. In addition, Nb and Cr additions
help form and stabilize alumina by increasing precipitate volume
fractions of NiAl.
Research endeavors for the development of alumina-forming,
Fe-base superalloys have shown at least 5 grades of alumina-forming
austenitic (AFA) alloys, with different operating temperatures at
oxidation in air + 10% water vapor:
- AFA Grade: (50-60)Fe-(20-25)Ni-(14-15)Cr-(2.5-3.5)Al-(1-3)Nb wt.% base
- 750-800 °C operating temperatures at oxidation in air + 10% water vapor
- Low Nickel AFA Grade: 63Fe-12Ni-14Cr-2.5Al-0.6Nb-5Mn3Cu wt.% base
- 650 °C operating temperatures at oxidation in air + 10% water vapor
- High Performance AFA Grade: (45-55)Fe-(25-30)Ni-(14-15)Cr(3.5-4.5)Al-(1-3)Nb-(0.02-0.1)Hf/Y wt.% base
- 850-900 °C operating temperatures at oxidation in air + 10% water vapor
- Cast AFA Grade: (35-50)Fe-(25-35)Ni-14Cr-(3.5-4)Al-1Nb wt.% base
- 750-1100 °C operating temperatures at oxidation in air + 10% water vapor, depending upon Ni wt.%
- AFA superalloy (40-50)Fe-(30-35)Ni-(14-19)Cr-(2.5-3.5)Al-3Nb
- 750-850 °C operating temperatures at oxidation in air + 10% water vapor
Operating temperatures with oxidation in air and no water vapor are
expected to be higher. In addition, an AFA superalloy grade was shown to
exhibit a creep strength approaching that of the nickel-based alloy UNS
N06617.
Microstructure of superalloys
In pure Ni3Al phase atoms
of aluminium are placed at the vertices of the cubic cell and form the
sublattice A. Atoms of nickel are located at centers of the faces and
form the sublattice B. The phase is not strictly stoichiometric.
There may exist an excess of vacancies in one of the sublattices, which
leads to deviations from stoichiometry. Sublattices A and B of the
γ'-phase can solute a considerable proportion of other elements. The
alloying elements are dissolved in the γ-phase as well. The γ'-phase
hardens the alloy through an unusual mechanism called the yield strength anomaly. Dislocations dissociate in the γ'-phase, leading to the formation of an anti-phase boundary.
At elevated temperature, the free energy associated with the anti-phase
boundary (APB) is considerably reduced if it lies on a particular
plane, which by coincidence is not a permitted slip plane. One set of
partial dislocations bounding the APB cross-slips so that the APB lies
on the low-energy plane, and, since this low-energy plane is not a
permitted slip plane, the dissociated dislocation is now effectively
locked. By this mechanism, the yield strength of γ'-phase Ni3Al actually increases with temperature up to about 1000 °C, giving superalloys their currently unrivaled high-temperature strength.
Initial material selection for blade applications in Gas Turbine engines included alloys like the Nimonic series alloys in the 1940s. The early Nimonic series incorporated γ' Ni3(Al,Ti) precipitates in a γ matrix, as well as various metal-carbon carbides (e.g. Cr23C6) at the grain boundaries for additional grain boundary strength. Turbine blade components were forged until vacuum induction casting technologies were introduced in the 1950s.
This process significantly improved cleanliness, reduced defects, and
increased the strength and temperature capability of the material.
Modern superalloys were developed in the 1980s. The first generation superalloys incorporated increased aluminium, titanium, tantalum, and niobium
content in order to increase the γ' volume fraction in these alloys.
Examples of first generation superalloys include: PWA1480, René N4 and
SRR99. Additionally, the volume fraction
of the γ' precipitates increased to about 50–70% with the advent of
single crystal, or monocrystal, solidification techniques (see Bridgman technique)
for superalloys that enable grain boundaries to be entirely eliminated
from a casting. Because the material contained no grain boundaries,
carbides were unnecessary as grain boundary strengthers and were thus
eliminated.
The second and third generation superalloys introduced about 3 and 6 weight per cent Rhenium,
for increased temperature capability. Re is a slow diffuser and
typically partitions to the γ matrix, decreasing the rate of diffusion
(and thereby high temperature creep)
and improving high temperature performance and increasing service
temperatures by 30 °C and 60 °C in second and third generation
superalloys, respectively.
Re has also been shown to promote the formation of rafts of the γ'
phase (as opposed to cuboidal precipitates). The presence of rafts can
decrease creep rate in the power-law regime
(controlled by dislocation climb), but can also potentially increase
the creep rate if the dominant mechanism is particle shearing.
Furthermore, Re tends to promote the formation of brittle TCP
phases, which has led to the strategy of reducing Co, W, Mo, and
particularly Cr. Younger generations of Ni-based superalloys have
significantly reduced Cr content for this reason, however with the
reduction in Cr comes a reduction in oxidation resistance. Advanced coating techniques are now used to offset the loss of oxidation resistance accompanying the decreased Cr contents. Examples of second generation superalloys include PWA1484, CMSX-4 and
René N5. Third generation alloys include CMSX-10, and René N6. Fourth,
Fifth, and even Sixth generation superalloys have been developed which
incorporate Ruthenium
additions, making them more expensive still than the prior generation's
Re-containing alloys. The effect of Ru on the promotion of TCP phases
is not well-determined. Early reports determined that Ru decreased the
supersaturation of Re in the matrix and thereby diminished the
susceptibility to TCP phase formation.
More recent studies have noted the opposite effect. Chen, et al., found
that in two alloys differing significantly only in Ru content (USTB-F3
and USTB-F6) that the addition of Ru increased both the partitioning
ratio as well as the supersaturation in the γ matrix of Cr and Re, and
thereby promoted the formation of TCP phases.
The current trend is to avoid very expensive and very heavy elements. An example is Eglin steel,
a budget material with compromised temperature range and chemical
resistance. It does not contain rhenium or ruthenium and its nickel
content is limited. To reduce fabrication costs, it was chemically
designed to melt in a ladle (though with improved properties in a vacuum
crucible). Also, conventional welding and casting is possible before
heat-treatment. The original purpose was to produce high-performance,
inexpensive bomb casings, but the material has proven widely applicable
to structural applications, including armor.
Single-crystal superalloys
Single-crystal superalloys (SX or SC superalloys) are formed as a single crystal using a modified version of the directional solidification technique, so there are no grain boundaries
in the material. The mechanical properties of most other alloys depend
on the presence of grain boundaries, but at high temperatures, they
would participate in creep and must be replaced by other mechanisms. In many such alloys, islands of an ordered intermetallic phase sit in a matrix of disordered phase, all with the same crystalline lattice. This approximates the dislocation-pinning behavior of grain boundaries, without introducing any amorphous solid into the structure.
Single crystal (SX) superalloys have wide application in the
high-pressure turbine section of aero and industrial gas turbine engines
due to the unique combination of properties and performance. Since
introduction of single crystal casting technology, SX alloy development
has focused on increased temperature capability, and major improvements
in alloy performance have been associated with the introduction of new
alloying elements, including rhenium (Re) and ruthenium (Ru).
With increasing turbine entry temperature, it is important to
gain a fundamental understanding of the physical phenomena occurring
during creep deformation of single crystal superalloys under such
extreme condition (i.e. high temperature and high stress). The creep
deformation behavior of superalloy single crystal is strongly
temperature, stress, orientation and alloy dependent. For a
single-crystal superalloy, there are 3 different mode of creep
deformation under regimes of different temperature and stress: Rafting,
Tertiary and Primary.
At low temperature (~750 °C), SX alloys exhibits mostly primary creep
behavior. Matan et al. concluded that the extent of primary creep
deformation depends strongly on the angle between the tensile axis and
the <001>/<011> symmetry boundary. At temperature above 850 °C, tertiary creep dominates and promotes strain softening behavior.
When temperature exceeds 1000 °C, the rafting effect is prevalent where
cubic particles transform into flat shapes under tensile stress
The rafts would also form perpendicular to the tensile axis, since γ
phase was transported out of the vertical channels and into the
horizontal ones. After conducting unaxial creep deformation of
<001> orientated CMSX-4 single crystal superalloy at 1105 °C and
100 MPa, Reed et al. has established that rafting is beneficial to creep
life since it delays evolution of creep strain. In addition, rafting
would occur quickly and suppress the accumulation of creep strain until a
critical strain is reached.
Oxidation in superalloys
For superalloys operating at high temperatures and exposed to corrosive environments, the oxidation behavior is of paramount concern. Oxidation involves chemical reactions of the alloying elements with oxygen to form new oxide
phases, generally at the surface of the metal. If unmitigated,
oxidation can degrade the alloy over time in a variety of ways,
including:
- sequential oxidation, cracking, and spalling of the surface, leading to erosion of the alloy over time.
- embrittlement of the surface through the introduction of oxide phases, promoting crack formation and fatigue failure
- depletion of key alloying elements, affecting the mechanical properties of the superalloy and possibly compromising its performance.
The primary strategy used to limit these deleterious processes is
called selective oxidation. Simply, the alloy is designed such that the
ratio of alloying elements promotes formation of a specific oxide phase
that can then act as a barrier to further oxidation. Most commonly, aluminum and chromium are used in this role, because they form relatively thin and continuous oxide layers of alumina (Al2O3) and chromia (Cr2O3), respectively. Furthermore, they possess low oxygen diffusivities,
effectively halting further oxidation beneath this layer. In the ideal
case, oxidation proceeds through 2 stages. First, transient oxidation
involves the conversion of various elements, especially the majority
elements (e.g. nickel or cobalt). Transient oxidation proceeds until the
selective oxidation of the sacrificial element forms a complete barrier
layer.
The protective effect of selective oxidation can be undermined by
numerous mechanisms. The continuity of the thin sacrificial oxide layer
can be compromised by mechanical disruption due to stress or may be disrupted as a result of the kinetics
of oxidation (e.g. if diffusion of oxygen is too fast). If the layer is
not continuous, its effectiveness as a diffusion barrier to oxygen is
significantly reduced. The stability of the oxide layer is also strongly
influenced by the presence of other minority elements. For example, the
addition of boron, silicon, and yttrium to superalloys promotes oxide layer adhesion, reducing spalling and maintaining the integrity of the protective oxide layer.
It should be noted that oxidation is only the most basic form of chemical degradation superalloys may experience. More complex corrosion
processes are common when operating environments include salts and
sulfur compounds, or under chemical conditions that change dramatically
over time. These issues and those of basic oxidation are often also
addressed through thin coatings.
Superalloy processing
Processing methods used to generate various portions of a jet turbine engine.
The historical developments in superalloy processing have brought about considerable increases in superalloy operating temperatures. Superalloys were originally iron based and cold wrought prior to the 1940s. In the 1940s investment casting of cobalt base alloys significantly raised operating temperatures. The development of vacuum melting
in the 1950s allowed for very fine control of the chemical composition
of superalloys and reduction in contamination and in turn led to a
revolution in processing techniques such as directional solidification of alloys and single crystal superalloys.
There are many forms of superalloy present within a gas turbine
engine, and processing methods vary widely depending on the necessary
properties of each specific part.
Casting and forging
Casting
and forging are traditional metallurgical processing techniques that
can be used to generate both polycrystalline and monocrystalline
products. Polycrystalline casts tend to have higher fracture resistance,
while monocrystalline casts have higher creep resistance.
Jet turbine engines employ both poly and mono crystalline
components to take advantage of their individual strengths. The disks of
the high-pressure turbine, which are near the central hub of the engine
are polycrystalline. The turbine blades, which extend radially into the
engine housing, experience a much greater centripetal force,
necessitating creep resistance. As a result, turbine blades are
typically monocrystalline or polycrystalline with a preferred crystal
orientation.
Investment casting
Investment casting
is a metallurgical processing technique in which a wax form is
fabricated and used as a template for a ceramic mold. Briefly, a ceramic
mold is poured around the wax form, the wax form is melted out of the
ceramic mold, and molten metal is poured into the void left by the wax.
This leads to a metal form in the same shape as the original wax form.
Investment casting leads to a polycrystalline final product, as
nucleation and growth of crystal grains occurs at numerous locations
throughout the solid matrix. Generally, the polycrystalline product has
no preferred grain orientation.
Schematic of directional solidification.
Directional solidification
Directional solidification
uses a thermal gradient to promote nucleation of metal grains on a low
temperature surface, as well as to promote their growth along the
temperature gradient. This leads to grains elongated along the
temperature gradient, and significantly greater creep resistance
parallel to the long grain direction. In polycrystalline turbine blades,
directional solidification is used to orient the grains parallel to the
centripetal force.It is also known as dendritic solidification.
Single crystal growth
Single crystal growth
starts with a seed crystal which is used to template growth of a larger
crystal. The overall process is lengthy, and additional processing via
machining is necessary after the single crystal is grown.
Powder metallurgy
Powder metallurgy
is a class of modern processing techniques in which metals are first
converted into a powder form, and then formed into the desired shape by
heating below the melting point. This is in contrast to casting, which
occurs with molten metal. Superalloy manufacturing often employs powder
metallurgy because of its material efficiency - typically much less
waste metal must be machined away from the final product—and its ability
to facilitate mechanical alloying. Mechanical alloying
is a process by which reinforcing particles are incorporated into the
superalloy matrix material by repeated fracture and welding.[36][not in citation given]
Sintering and hot isostatic pressing
Sintering and hot isostatic pressing are processing techniques used to densify materials from a loosely packed "green body"
into a solid object with physically merged grains. Sintering occurs
below the melting point, and causes adjacent particles to merge at their
boundaries, leading to a strong bond between them. In hot isostatic
pressing, a sintered material is placed in a pressure vessel and
compressed from all directions (isostatically) in an inert atmosphere to
affect densification.
Additive manufacturing
Selective
Laser Melting (also known as Powder Bed Fusion) is an additive
manufacturing procedure used to create intricately detailed forms from a
CAD file. In CAD, a shape is designed and then converted into slices.
These slices are sent to a laser writer to print the final product. In
brief, a bed of metal powder is prepared, and the first slice of the CAD
design is formed in the powder bed by a high energy laser sintering the
particles together. After this first slice is generated, the powder bed
moves downwards, and a new batch of metal powder is rolled over the top
of the slice. The second layer is then sintered with the laser, and the
process is repeated until all the slices in the CAD file have been
processed.
Coating of superalloys
In
modern gas turbine, the turbine entry temperature (~1750K) has exceeded
the incipient melting temperature of superalloys (~1600K), with the
help of surface engineering. Under such extreme working condition, the
qualification of coating becomes vital.
Different types of coating
Historically,
three "generations" of coatings have been developed: diffusion
coatings, overlay coatings and thermal barrier coatings. Diffusion
coatings, mainly constituted with aluminide or platinum-aluminide, is
still the most common form of surface protection. To further enhance
resistance to corrosion and oxidation, MCrAlX-based overlay coatings
(M=Ni or Co, X=Y, Hf, Si) are deposited to surface of superalloys.
Compared to diffusion coatings, overlay coatings are less dependent on
the composition of the substrate, but also more expensive, since they
must be carried out by air or vacuum plasma spraying (APS/VPS) or else electron beam physical vapour deposition (EB-PVD).
Thermal barrier coatings provide by far the best enhancement in working
temperature and coating life. It is estimated that modern TBC of
thickness 300 μm, if used in conjunction with a hollow component and
cooling air, has the potential to lower metal surface temperatures by a
few hundred degrees.
Thermal barrier coatings
Thermal
barrier coatings (TBCs) are used extensively on the surface of
superalloy in both commercial and military gas turbine engines to
increase component life and engine performance.
A coating of about 1-200 µm can reduce the temperature at the
superalloy surface by up to 200K. TBCs are really a system of coatings
consisting of a bond coat, a thermally grown oxide (TGO), and a
thermally insulating ceramic top coat. In most applications, the bond
coat is either a MCrAlY (where M=Ni or NiCo) or a Pt modified aluminide
coating. A dense bond coat is required to provide protection of the
superalloy substrate from oxidation and hot corrosion attack and to form
an adherent, slow growing TGO on its surface. The TGO is formed by
oxidation of the aluminum that is contained in the bond coat. The
current (first generation) thermal insulation layer is composed of 7wt %
yttria-stabilized zirconia
(7YSZ) with a typical thickness of 100–300 µm. Yttria stabilized
zirconia is used due to its low thermal conductivity (2.6W/mK for fully
dense material), relatively high coefficient of thermal expansion, and
good high temperature stability. The electron beam directed vapor
deposition (EB-DVD) process used to apply the TBC to turbine airfoils
produces a columnar microstructure with several levels of porosity. The
porosity between the columns is critical to providing strain tolerance
(via a very low in-plane modulus), as it would otherwise spall on
thermal cycling due to thermal expansion mismatch with the superalloy
substrate. The porosity within the columns reduces the thermal
conductivity of the coating.
Bond coat
The
bond coat adheres the thermal barrier coating to the superalloy
substrate. Additionally, the bond coat provides oxidation protection and
functions as a diffusion barrier against the motion of substrate atoms
towards the environment.
There are five major types of bond coats, the aluminides, the
platinum-aluminides, MCrAlY, cobalt-cermets, and nickel-chromium.
For the aluminide bond coatings, the final composition and structure of
the coating depends on the composition of the substrate. Aluminides also
lack ductility below 750 °C, and exhibit a limited by thermomechanical
fatigue strength. The Pt-aluminides are very similar to the aluminide
bond coats except for a layer of Pt (5—10 μm) deposited on the blade.
The Pt is believed to aid in oxide adhesion and contributes to hot
corrosion. The cost of Pt plating is justified by the increased blade
life span. The MCrAlY is the latest generation of bond coat and does not
strongly interact with the substrate. Normally applied by plasma
spraying, MCrAlY coatings are secondary aluminum oxide formers. This
means that the coatings form an outer layer of chromium oxide (chromia),
and a secondary aluminum oxide (alumina) layer underneath. These oxide
formations occur at high temperatures in the range of those that
superalloys usually encounter.
The chromia provides oxidation and hot-corrosion resistance. The
alumina controls oxidation mechanisms by limiting oxide growth by
self-passivating. The yttrium enhances the oxide adherence to the
substrate, and limits the growth of grain boundaries (which can lead to
flaking of the coating). Investigation indicates that addition of rhenium and tantalum increases oxidation resistance. Cobalt-cermet based coatings consisting of materials such as tungsten carbide/cobalt can be used due to excellent resistance to abrasion, corrosion, erosion, and heat. These cermet
coatings perform well in situations where temperature and oxidation
damage are significant concerns, such as boilers. One of the unique
advantages of cobalt cermet coatings is a minimal loss of coating mass
over time, due to the strength of carbides within the mixture. Overall,
cermet coatings are useful in situations where mechanical demands are
equal to chemical demands for superalloys. Nickel-chromium coatings are
used most frequently in boilers fed by fossil fuels, electric furnaces, and waste incineration furnaces, where the danger of oxidizing agents and corrosive compounds in the vapor must be dealt with.
The specific method of spray-coating depends on the composition of the
coatings. Nickel-chromium coatings that also contain iron or aluminum
perform much better (in terms of corrosion resistance) when they are
sprayed and laser glazed, while pure nickel-chromium coatings perform
better when thermally sprayed exclusively.
Process methods of coating
Superalloy
products that are subjected to high working temperatures and corrosive
atmosphere (such as high-pressure turbine region of jet engines) are
coated with various kinds of coating. Several kinds of coating process are applied: pack cementation process, gas phase coating (both are a type of chemical vapor deposition (CVD)), thermal spraying, and physical vapor deposition.
In most cases, after the coating process near-surface regions of parts
are enriched with aluminium, the matrix of the coating being nickel aluminide.
Pack cementation process
The
pack cementation process is carried out at lower temperatures, about
750 °C. The parts are loaded into boxes that contain a mixture of
powders: active coating material, containing aluminum, activator (chloride or fluoride), and thermal ballast, like aluminum oxide. At high temperatures the gaseous aluminum chloride
is transferred to the surface of the part and diffuses inside (mostly
inward diffusion). After the end of the process the so-called "green
coating" is produced, which is too thin and brittle for direct use. A
subsequent diffusion heat treatment (several hours at temperatures about
1080 °C) leads to further inward diffusion and formation of the desired
coating.
Thermal spraying
Thermal
spraying is a process of applying coatings by heating a feedstock of
precursor material and spraying it on a surface. Different specific
techniques are used depending on desired particle size, coat thickness,
spray speed, desired area, etc.[49][full citation needed]
The coatings applied by thermal spraying of any kind, however, rely on
adhesion to the surface. As a result, the surface of the superalloy must
be cleaned and prepared, usually polished, before application of the
thermal coating.
Plasma spraying
Of the various thermal spray methods, one of the more ideal and commonly used techniques for coating superalloys is plasma spraying. This is due to the versatility of usable coatings, and the high-temperature performance of plasma-sprayed coatings.
Plasma spraying can accommodate a very wide range of materials, much
more so than other techniques. As long as the difference between melting
and decomposition temperatures is greater than 300 Kelvin, a material
can be melted and applied as a coating via plasma spraying.
Gas phase coating
This
process is carried out at higher temperatures, about 1080 °C. The
coating material is usually loaded onto special trays without physical
contact with the parts to be coated. The coating mixture contains active
coating material and activator, but usually does not contain thermal
ballast. As in the pack cementation process, the gaseous aluminium
chloride (or fluoride) is transferred to the surface of the part.
However, in this case the diffusion is outwards. This kind of coating
also requires diffusion heat treatment.
Failure mechanisms in thermal barrier coating systems
Failure
of thermal barrier coating usually manifests as delamination, which
arises from the temperature gradient during thermal cycling between
ambient temperature and working conditions coupled with the difference
in thermal expansion coefficient of the substrate and coating. It is
rare for the coating to fail completely – some pieces of it remain
intact, and significant scatter is observed in the time to failure if
testing is repeated under identical conditions. There are various degradation mechanisms for thermal barrier coating, and some or all of these must operate before failure finally occurs:
- Oxidation at the interface of thermal barrier coating and underlying bond coat;
- The depletion of aluminum in bond coat due to oxidation and diffusion with substrate;
- Thermal stresses from mismatch in thermal expansion coefficient and growth stress due to the formation of thermally grown oxide layer;
- Imperfections near thermally grown oxide layer;
- Various other complicating factors during engine operation.
Additionally, TBC life is very dependent upon the combination of
materials (substrate, bond coat, ceramic) and processes (EB-PVD, plasma
spraying) used.
Applications
Nickel-based
superalloys are used in load-bearing structures to the highest
homologous temperature of any common alloy system (Tm = 0.9, or 90% of
their melting point). Among the most demanding applications for a
structural material are those in the hot sections of turbine engines.
The preeminence of superalloys is reflected in the fact that they
currently comprise over 50% of the weight of advanced aircraft engines.
The widespread use of superalloys in turbine engines coupled with the
fact that the thermodynamic efficiency of turbine engines is increased
with increasing turbine inlet temperatures has, in part, provided the
motivation for increasing the maximum-use temperature of superalloys. In
fact, during the past 30 years turbine airfoil temperature capability
has increased on average by about 4 °F (2.2 °C) per year. Two major
factors which have made this increase possible are
- Advanced processing techniques, which improved alloy cleanliness (thus improving reliability) and/or enabled the production of tailored microstructures such as directionally solidified or single-crystal material.
- Alloy development resulting in higher-use-temperature materials primarily through the additions of refractory elements such as Re, W, Ta, and Mo.
About 60% of the use-temperature increases have occurred due to
advanced cooling concepts; 40% have resulted from material improvements.
State-of-the-art turbine blade surface temperatures are near 2,100 °F
(1,150 °C); the most severe combinations of stress and temperature
corresponds to an average bulk metal temperature approaching 1,830 °F
(1,000 °C).
Although superalloys retain significant strength to temperatures
near 1,800 °F (980 °C), they tend to be susceptible to environmental
attack because of the presence of reactive alloying elements (which
provide their high-temperature strength). Surface attack includes
oxidation, hot corrosion, and thermal fatigue. In the most demanding
applications, such as turbine blade and vanes, superalloys are often
coated to improve environmental resistance.
In general, high temperature materials are needed for energy
conversion and energy production applications. Maximum energy conversion
efficiency is desired in these energy applications, which can be
achieved by increasing operating temperatures, as described by the
Carnot cycle. Operating temperatures are limited by the performance of
today’s superalloys, and currently, most applications operate at around
1000oC-1400 oC. Energy applications and their superalloy components include:
- Gas turbines (turbine blades)
- Solar thermal power plants (stainless steel rods containing heated water)
- Steam turbines (turbine blades and boiler housing)
- Heat exchangers for nuclear reactor systems.
Alumina-forming stainless steels can be processed via melting and
ladle casting, similar to the production of more common steels. Unlike
vacuum casting processes, ladle casting is much more inexpensive. In
addition, alumina-forming stainless steel has been shown to be weldable
and has potential for use in high performance automotive applications,
such as for high temperature exhaust piping and in heat capture and
reuse.
Research and development of new superalloys
The
availability of superalloys during past decades has led to a steady
increase in the turbine entry temperatures and the trend is expected to
continue. Sandia National Laboratories is studying a new method for making superalloys, known as radiolysis. It introduces an entirely new area of research into creating alloys and superalloys through nanoparticle synthesis. This process holds promise as a universal method of nanoparticle formation. By developing an understanding of the basic material science
behind these nanoparticle formations, there is speculation that it
might be possible to expand research into other aspects of superalloys.
There may be considerable disadvantages in making alloys by this
method. About half of the use of superalloys is in applications where
the service temperature is close to the melting temperature of the
alloy. It is common therefore to use single crystals. The above method
produces polycrystalline alloys, which suffer from an unacceptable level
of creep.
Future paradigm in alloy development focus on reduction of
weight, improving oxidation and corrosion resistance while maintaining
the strength of the alloy. Furthermore, with the increasing demand for
turbine blade for power generation, another focus of alloy design is to
reduce the cost of super alloys.
There has been ongoing research and development of new stainless
steel alloys because of the lower costs in producing these alloys as
well as the need for an austenitic stainless steel with high temperature
corrosion resistance in environments with water vapor. Research is
focused on increasing high temperature tensile strength, toughness, and
creep resistance to compete with Ni-based superalloys.
A new class of alumina-forming austenitic stainless steel is
actively being developed for use in high-temperature applications by Oak
Ridge National Laboratory. Initial research showed similar creep and
corrosion resistance at 800oC to that of other austenitic alloys, including Ni-based superalloys.
Development of AFA superalloys with a 35 wt.% Ni-base have shown
potential for use in operating temperatures upwards to 1,100 °C.