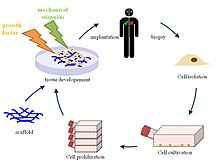
Principle of tissue engineering
Decellularization (also spelt decellularisation in British English) is the process used in biomedical engineering to isolate the extracellular matrix (ECM) of a tissue from its inhabiting cells, leaving an ECM scaffold of the original tissue, which can be used in artificial organ and tissue regeneration. Organ
and tissue transplantation treat a variety of medical problems, ranging
from end organ failure to cosmetic surgery. One of the greatest
limitations to organ transplantation derives from organ rejection caused
by antibodies of the transplant recipient reacting to donor antigens on cell surfaces within the donor organ. Because of unfavorable immune
responses, transplant patients suffer a lifetime taking
immunosuppressing medication. Stephen F. Badylak pioneered the process
of decellularization at the McGowan Institute for Regenerative Medicine
at the University of Pittsburgh.[2] This process creates a natural biomaterial to act as a scaffold for cell growth, differentiation
and tissue development. By recellularizing an ECM scaffold with a
patient’s own cells, the adverse immune response is eliminated.
Nowadays, commercially available ECM scaffolds are available for a wide
variety of tissue engineering. Using peracetic acid to decellularize ECM scaffolds have been found to be false and only disinfects the tissue.
With a wide variety of decellularization-inducing treatments available, combinations of physical, chemical,
and enzymatic treatments are carefully monitored to ensure that the ECM
scaffold maintains the structural and chemical integrity of the
original tissue. Scientists can use the acquired ECM scaffold to reproduce a functional organ by introducing progenitor cells, or adult stem cells
(ASCs), and allowing them to differentiate within the scaffold to
develop into the desired tissue. The produced organ or tissue can be
transplanted into a patient. In contrast to cell surface antibodies, the
biochemical components of the ECM are conserved between hosts, so the risk of a hostile immune response is minimized.
Proper conservation of ECM fibers, growth factors, and other proteins
is imperative to the progenitor cells differentiating into the proper
adult cells. The success of decellularization varies based on the
components and density of the applied tissue and its origin. The applications to the decellularizing method of producing a biomaterial scaffold for tissue regeneration are present in cardiac, dermal, pulmonary, renal, and other types of tissues. Complete organ reconstruction is still in the early levels of development.
Process overview
Researchers are able to take the tissue from a donor or cadaver, lyse
and kill the cells within the tissue without damaging the extracellular
components, and finish with a product that is the natural ECM scaffold
that has the same physical and biochemical functions of the natural
tissue. After acquiring the ECM scaffold, scientists can recellularize the tissue with potent stem
or progenitor cells that will differentiate into the original type of
tissue. By removing the cells from a donor tissue, the immunogenic
antibodies from the donor will be removed. The progenitor cells can be
taken from the host, therefore they will not have an adverse response to
the tissue. This process of decellularizing tissues and organs is
still being developed, but the exact process of taking a tissue from a
donor and removing all the cellular components is considered to be the
decellularization process. The steps to go from a decellularized ECM
scaffold to a functional organ is under the umbrella of
recellularization. Because of the diverse applications of tissue in the
human body, decellularization techniques have to be tailored to the
specific tissue being exercised on. The researched methods of
decellularization include physical, chemical, and enzymatic treatments.
Though some methods are more commonly used, the exact combination of
treatments is variable based on the tissue’s origin and what it is
needed for.
As far as introducing the different liquidized chemicals and enzymes to an organ or tissue, perfusion
and immersion decellularization techniques have been used. Perfusion
decellularization is applicable when an extensive vasculature system is
present in the organ or tissue. It is crucial for the ECM scaffold to be
decellularized at all levels, and evenly throughout the structure.
Because of this requirement, vascularized tissues can have chemicals
and enzymes perfused through the present arteries, veins, and
capillaries. Under this mechanism and proper physiological conditions,
treatments can diffuse equally to all of the cells within the organ. The
treatments can be removed through the veins at the end of the process.
Cardiac and pulmonary decellularization often uses this process of
decellularization to introduce the treatments because of their heavily
vascularized networks. Immersion decellularization is accomplished
through the submersion of a tissue in chemical and enzymatic treatments.
This process is more easily accomplished than perfusion, but is limited to thin tissues with a limited vascular system.
Physical treatments
The
most common physical methods used to lyse, kill, and remove cells from
the matrix of a tissue through the use of temperature, force and
pressure, and electrical disruption. Temperature methods are often used
in a rapid freeze-thaw mechanism. By quickly freezing a tissue,
microscopic ice crystals form around the plasma membrane and the cell is
lysed.
After lysing the cells, the tissue can be further exposed to liquidized
chemicals that degrade and wash out the undesirable components.
Temperature methods conserve the physical structure of the ECM scaffold,
but are best handled by thick, strong tissues.
Direct force of pressure to a tissue will guarantee disruption of
the ECM structure, so pressure is commonly used. Pressure
decellularization involves the controlled use of hydrostatic pressure
applied to a tissue or organ. This is done best at high temperatures
to avoid unmonitored ice crystal formation that could damage the
scaffold. Electrical disruption of the plasma membrane is another option
to lyse the cells housed in a tissue or organ. By exposing a tissue to
electrical pulses, micropores are formed at the plasma membrane. The
cells eventually turn to death after their homeostatic electrical
balance is ruined through the applied stimulus. This electrical process
is documented as Non-thermal irreversible electroporation (NTIRE) and is limited to small tissues and the limited possibilities of inducing an electric current in vivo.
Chemical treatments
The
proper combination of chemicals is selected for decellularization
depending on the thickness, extracellular matrix composition, and
intended use of the tissue or organ. For example, enzymes would not be
used on a collagenous tissue because they disrupt the connective tissue
fibers. However, when collagen
is not present in a high concentration or needed in the tissue, enzymes
can be a viable option for decellularization. The chemicals used to
kill and remove the cells include acids, alkaline treatments, ionic detergents, non-ionic detergents, and zwitterionic detergents.
The ionic detergent, sodium dodecyl sulfate (SDS), is commonly used because of its high efficacy for lysing cells without significant damage to the ECM.
Detergents act effectively to lyse the cell membrane and expose the
contents to further degradation. After SDS lyses the cell membrane, endonucleases and exonucleases
degrade the genetic contents, while other components of the cell is
solubilized and washed out of the matrix. SDS is commonly used even
though it has a tendency to slightly disrupt the ECM structure. Akaline
and acid treatments can be effective companions with an SDS treatment
due to their ability to degrade nucleic acids and solubilize cytoplasmic inclusions.
The most well known non-ionic detergent is Triton X-100, which is popular because of its ability to disrupt the interactions between lipids and between lipids and proteins. Triton X-100 does not disrupt protein-protein interactions, which is beneficial to keeping the ECM intact. EDTA is a chelating agent that binds calcium, which is a necessary component for proteins to interact with one another. By making calcium unavailable, EDTA
prevents the integral proteins between cells from binding to one
another. EDTA is often used with trypsin, an enzyme that acts as a
protease to cleave the already existing bonds between integral proteins
of neighboring cells within a tissue. Together, the EDTA-Trypsin
combination make a good team for decellularizing tissues.
Enzymatic treatments
Enzymes
used in decellularization treatments are used to break the bonds and
interactions between nucleic acids, interacting cells through
neighboring proteins, and other cellular components. Lipases, thermolysin, galactosidase, nucleases, and trypsin
have all been used in the removal of cells. After a cell is lysed with
a detergent, acid, physical pressure, etc., endonucleases and
exonucleases can begin the degradation of the genetic material.
Endonucleases cleave DNA and RNA in the middle of sequences. Benzoase,
an endonuclease, produces multiple small nuclear fragments that can be
further degraded and removed from the ECM scaffold.
Exonucleases act at the end of DNA sequences to cleave the
phosphodiester bonds and further degrade the nucleic acid sequences.
Enzymes such as trypsin act as proteases that cleave the
interactions between proteins. Although trypsin can have adverse effects
of collagen and elastin fibers of the ECM, using it in a time-sensitive
manner controls any potential damage it could cause on the
extracellular fibers. Dispase is used to prevent undesired aggregation
of cells, which is beneficial in promoting their separating from the ECM
scaffold. Experimentation has shown dispase to be most effective on
the surface of a thin tissue, such as a lung in pulmonary tissue
regeneration. To successfully remove deep cells of a tissue with
dispase, mechanical agitation is often included in the process.
Collagenase
is only used when the ECM scaffold product does not require an intact
collagen structure. Lipases are commonly used when decellularized skin
grafts are needed. Lipase acids function in decellularizing dermal
tissues through delipidation and cleaving the interactions between
heavily lipidized cells. The enzyme, α-galactosidase is a relevant
treatment when removing the Gal epitope antigen from cell surfaces.
Applications
A natural ECM scaffold provides the necessary physical and
biochemical environment to facilitate the growth and specialization of
potent progenitor and stem cells. Acellular matrices have been isolated
in vitro and in vivo in a number of different tissues and organs.
The most applicable success from decellularized tissues has come from
symmetrical tissues that have less specialization, such as bone and
dermal grafts; however, research and success is ongoing at the organ
level.
Acellular dermal matrices have been successful in a number of
different applications. For example, skin grafts are used in cosmetic
surgery and burn care. The decellularized skin graft provides
mechanical support to the damaged area while supporting the development
of host-derived connective tissue. Cardiac tissue has clinical success
in developing human valves from natural ECM matrices.[14]
A technique known as the Ross procedure uses an acellular heart valve
to replace a defective valve, allowing native cells to repopulate a
newly functioning valve. Decellularized allografts have been critical in bone grafts that function in bone reconstruction and replacing of deformed bones in patients.
The limits to myocardial tissue engineering come from the ability
to immediately perfuse and seed and implemented heart into a patient.
Though the ECM scaffold maintains the protein and growth factors
of the natural tissue, the molecular level specialization has not yet
been harnessed by researchers using decellularized heart scaffolds.
Better success at using a whole organ from decellularization techniques
has been found in pulmonary research. Scientists have been able to
regenerate whole lungs in vitro from rat lungs using perfusion-decellularization. By seeding the matrix with fetal rat lung cells, a functioning lung was produced. The in vitro-produced lung was successfully implemented into a rat, which attests to the possibilities of translating an in vitro produced organ into a patient.
Other success for decellularization has been found in small intestinal submucosa (SIS), renal, hepatic, and pancreatic engineering.
Because it is a thin material, the SIS matrix can be decellularized
through immersing the tissue in chemical and enzymatic treatments. Renal
tissue engineering is still developing, but cadaveric kidney matrices
have been able to support development of potent fetal kidney cells.
Pancreatic engineering is a testament to the molecular specificity of
organs. Scientists have not yet been able to produce an entirely
functioning pancreas,
but they have had success in producing an organ that functions at
specific segments. For example, diabetes in rats was shown to decrease
by seeding a pancreatic matrix at specific sites.
The future applications of decellularized tissue matrix is still being
discovered and is considered one of the most hopeful areas in
regenerative research.