Latex being collected from a tapped rubber tree, Cameroon
Rubber tree plantation in Thailand
Natural rubber, also called India rubber or caoutchouc, as initially produced, consists of polymers of the organic compound isoprene, with minor impurities of other organic compounds, plus water. Malaysia and Indonesia are two of the leading rubber producers. Forms of polyisoprene that are used as natural rubbers are classified as elastomers.
Currently, rubber is harvested mainly in the form of the latex from the rubber tree or others. The latex is a sticky, milky colloid
drawn off by making incisions in the bark and collecting the fluid in
vessels in a process called "tapping". The latex then is refined into
rubber ready for commercial processing. In major areas, latex is
allowed to coagulate in the collection cup. The coagulated lumps are
collected and processed into dry forms for marketing.
Natural rubber is used extensively in many applications and
products, either alone or in combination with other materials. In most
of its useful forms, it has a large stretch ratio and high resilience, and is extremely waterproof.
Varieties
Hevea brasiliensis
The major commercial source of natural rubber latex is the Pará rubber tree (Hevea brasiliensis), a member of the spurge family, Euphorbiaceae.
This species is preferred because it grows well under cultivation. A
properly managed tree responds to wounding by producing more latex for
several years.
Congo rubber
Congo rubber, formerly a major source of rubber, came from vines in the genus Landolphia (L. kirkii, L. heudelotis, and L. owariensis).
Dandelion
Dandelion milk contains latex. The latex exhibits the same quality as the natural rubber from rubber trees. In the wild types of dandelion, latex content is low and varies greatly. In Nazi Germany, research projects tried to use dandelions as a base for rubber production, but failed. In 2013, by inhibiting one key enzyme and using modern cultivation methods and optimization techniques, scientists in the Fraunhofer Institute for Molecular Biology and Applied Ecology (IME) in Germany developed a cultivar that is suitable for commercial production of natural rubber. In collaboration with Continental Tires, IME began a pilot facility.
Other
Many other
plants produce forms of latex rich in isoprene polymers, though not all
produce usable forms of polymer as easily as the Pará. Some of them
require more elaborate processing to produce anything like usable
rubber, and most are more difficult to tap. Some produce other desirable
materials, for example gutta-percha (Palaquium gutta) and chicle from Manilkara species. Others that have been commercially exploited, or at least showed promise as rubber sources, include the rubber fig (Ficus elastica), Panama rubber tree (Castilla elastica), various spurges (Euphorbia spp.), lettuce (Lactuca species), the related Scorzonera tau-saghyz, various Taraxacum species, including common dandelion (Taraxacum officinale) and Russian dandelion (Taraxacum kok-saghyz), and perhaps most importantly for its hypoallergenic properties, guayule (Parthenium argentatum). The term gum rubber is sometimes applied to the tree-obtained version of natural rubber in order to distinguish it from the synthetic version.
History
The first use of rubber was by the indigenous cultures of Mesoamerica. The earliest archeological evidence of the use of natural latex from the Hevea tree comes from the Olmec culture, in which rubber was first used for making balls for the Mesoamerican ballgame. Rubber was later used by the Maya and Aztec
cultures – in addition to making balls Aztecs used rubber for other
purposes such as making containers and to make textiles waterproof by
impregnating them with the latex sap.
The Pará rubber tree is indigenous to South America. Charles Marie de La Condamine is credited with introducing samples of rubber to the Académie Royale des Sciences of France in 1736. In 1751, he presented a paper by François Fresneau
to the Académie (published in 1755) that described many of rubber's
properties. This has been referred to as the first scientific paper on
rubber. In England, Joseph Priestley, in 1770, observed that a piece of the material was extremely good for rubbing off pencil marks on paper, hence the name "rubber". It slowly made its way around England. In 1764 François Fresnau discovered that turpentine was a rubber solvent. Giovanni Fabbroni is credited with the discovery of naphtha as a rubber solvent in 1779.
South America remained the main source of the limited amounts of
latex rubber used during much of the 19th century. The trade was heavily
protected and exporting seeds from Brazil was a capital offense, although no law prohibited it. Nevertheless, in 1876, Henry Wickham smuggled 70,000 Pará rubber tree seeds from Brazil and delivered them to Kew Gardens, England. Only 2,400 of these germinated. Seedlings were then sent to India, British Ceylon (Sri Lanka), Dutch East Indies (Indonesia), Singapore, and British Malaya. Malaya (now Peninsular Malaysia) was later to become the biggest producer of rubber.
In the early 1900s, the Congo Free State in Africa was also a significant source of natural rubber latex, mostly gathered by forced labor.
King Leopold II's colonial state brutally enforced production quotas.
Tactics to enforce the rubber quotas included removing the hands of
victims to prove they had been killed. Soldiers often came back from
raids with baskets full of chopped-off hands. Villages that resisted
were razed to encourage better compliance locally. Liberia and Nigeria started production.
In India,
commercial cultivation was introduced by British planters, although the
experimental efforts to grow rubber on a commercial scale were
initiated as early as 1873 at the Calcutta Botanical Gardens. The first commercial Hevea plantations were established at Thattekadu in Kerala in 1902. In later years the plantation expanded to Karnataka, Tamil Nadu and the Andaman and Nicobar Islands of India. India today is the world's 3rd largest producer and 4th largest consumer.
In Singapore and Malaya, commercial production was heavily promoted by Sir Henry Nicholas Ridley, who served as the first Scientific Director of the Singapore Botanic Gardens
from 1888 to 1911. He distributed rubber seeds to many planters and
developed the first technique for tapping trees for latex without
causing serious harm to the tree. Because of his fervent promotion of this crop, he is popularly remembered by the nickname "Mad Ridley".
Pre-World War II
Charles Goodyear developed vulcanization in 1839, although Mesoamericans used stabilized rubber for balls and other objects as early as 1600 BC.
Before World War II significant uses included door and window profiles, hoses, belts, gaskets, matting, flooring and dampeners (antivibration mounts) for the automotive industry. The use of rubber in car tires (initially solid rather than pneumatic) in particular consumed a significant amount of rubber. Gloves (medical, household and industrial) and toy balloons
were large consumers of rubber, although the type of rubber used is
concentrated latex. Significant tonnage of rubber was used as adhesives
in many manufacturing industries and products, although the two most
noticeable were the paper and the carpet industries. Rubber was commonly
used to make rubber bands and pencil erasers.
Rubber produced as a fiber, sometimes called 'elastic', had
significant value to the textile industry because of its excellent
elongation and recovery properties. For these purposes, manufactured
rubber fiber was made as either an extruded round fiber or rectangular
fibers cut into strips from extruded film. Because of its low dye
acceptance, feel and appearance, the rubber fiber was either covered by
yarn of another fiber or directly woven with other yarns into the
fabric. Rubber yarns were used in foundation garments. While rubber is
still used in textile manufacturing, its low tenacity limits its use in
lightweight garments because latex lacks resistance to oxidizing agents
and is damaged by aging, sunlight, oil and perspiration. The textile
industry turned to neoprene (polymer of chloroprene), a type of synthetic rubber, as well as another more commonly used elastomer fiber, spandex (also known as elastane), because of their superiority to rubber in both strength and durability.
Properties
Rubber latex
Rubber exhibits unique physical and chemical properties. Rubber's stress–strain behavior exhibits the Mullins effect and the Payne effect and is often modeled as hyperelastic. Rubber strain crystallizes.
Due to the presence of weakened allylic C-H bonds in each repeat unit, natural rubber is susceptible to vulcanisation as well as being sensitive to ozone cracking.
The two main solvents for rubber are turpentine and naphtha (petroleum). Because rubber does not dissolve easily, the material is finely divided by shredding prior to its immersion.
An ammonia solution can be used to prevent the coagulation of raw latex.
Rubber begins to melt at approximately 180 °C (356 °F).
Elasticity
On a microscopic scale, relaxed rubber is a disorganized cluster of
erratically changing wrinkled chains. In stretched rubber, the chains
are almost linear. The restoring force is due to the preponderance of
wrinkled conformations over more linear ones. For the quantitative
treatment see ideal chain, for more examples see entropic force.
Cooling below the glass transition temperature
permits local conformational changes but a reordering is practically
impossible because of the larger energy barrier for the concerted
movement of longer chains. "Frozen" rubber's elasticity is low and strain results from small changes of bond lengths and angles: this caused the Challenger disaster, when the American Space Shuttle's flattened o-rings failed to relax to fill a widening gap. The glass transition is fast and reversible: the force resumes on heating.
The parallel chains of stretched rubber are susceptible to
crystallization. This takes some time because turns of twisted chains
have to move out of the way of the growing crystallites. Crystallization
has occurred, for example, when, after days, an inflated toy balloon is
found withered at a relatively large remaining volume. Where it is
touched, it shrinks because the temperature of the hand is enough to
melt the crystals.
Vulcanization of rubber creates di- and polysulfide bonds between chains, which limits the degrees of freedom
and results in chains that tighten more quickly for a given strain,
thereby increasing the elastic force constant and making the rubber
harder and less extensible.
Malodour
Raw
rubber storage depots and rubber processing can produce malodour that is
serious enough to become a source of complaints and protest to those
living in the vicinity.
Microbial impurities originate during the processing of block
rubber. These impurities break down during storage or thermal
degradation and produce volatile organic compounds. Examination of these
compounds using gas chromatography/mass spectrometry (GC/MS) and gas chromatography (GC) indicates that they contain sulphur, ammonia, alkenes, ketones, esters, hydrogen sulphite, nitrogen, and low molecular weight fatty acids (C2-C5).
When latex concentrate is produced from rubber, sulphuric acid is
used for coagulation. This produces malodourous hydrogen sulphide.
The industry can mitigate these bad odours with scrubber systems.
Chemical makeup
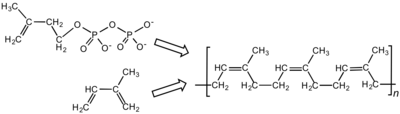
Chemical
structure of cis-polyisoprene, the main constituent of natural rubber.
Synthetic cis-polyisoprene and natural cis-polyisoprene are derived from
different precursors, isopentenyl pyrophosphate and isoprene.
Latex is the polymer cis-1,4-polyisoprene – with a molecular weight of 100,000 to 1,000,000 daltons. Typically, a small percentage (up to 5% of dry mass) of other materials, such as proteins, fatty acids, resins,
and inorganic materials (salts) are found in natural rubber.
Polyisoprene can also be created synthetically, producing what is
sometimes referred to as "synthetic natural rubber", but the synthetic
and natural routes are different. Some natural rubber sources, such as gutta-percha, are composed of trans-1,4-polyisoprene, a structural isomer that has similar properties.
Natural rubber is an elastomer and a thermoplastic. Once the rubber is vulcanized, it is a thermoset.
Most rubber in everyday use is vulcanized to a point where it shares
properties of both; i.e., if it is heated and cooled, it is degraded but
not destroyed.
The final properties of a rubber item depend not just on the polymer, but also on modifiers and fillers, such as carbon black, factice, whiting and others.
Biosynthesis
Rubber particles are formed in the cytoplasm of specialized latex-producing cells called laticifers within rubber plants. Rubber particles are surrounded by a single phospholipid membrane with hydrophobic
tails pointed inward. The membrane allows biosynthetic proteins to be
sequestered at the surface of the growing rubber particle, which allows
new monomeric units to be added from outside the biomembrane, but within
the lacticifer. The rubber particle is an enzymatically active entity
that contains three layers of material, the rubber particle, a
biomembrane and free monomeric units. The biomembrane is held tightly to
the rubber core due to the high negative charge along the double bonds
of the rubber polymer backbone. Free monomeric units and conjugated proteins make up the outer layer. The rubber precursor is isopentenyl pyrophosphate (an allylic compound), which elongates by Mg2+-dependent condensation by the action of rubber transferase. The monomer adds to the pyrophosphate end of the growing polymer.
The process displaces the terminal high-energy pyrophosphate. The
reaction produces a cis polymer. The initiation step is catalyzed by prenyltransferase, which converts three monomers of isopentenyl pyrophosphate into farnesyl pyrophosphate. The farnesyl pyrophosphate can bind to rubber transferase to elongate a new rubber polymer.
The required isopentenyl pyrophosphate is obtained from the mevalonate pathway, which derives from acetyl-CoA in the cytosol.
In plants, isoprene pyrophosphate can also be obtained from the
1-deox-D-xyulose-5-phosphate/2-C-methyl-D-erythritol-4-phosphate pathway
within plasmids.
The relative ratio of the farnesyl pyrophosphate initiator unit and
isoprenyl pyrophosphate elongation monomer determines the rate of new
particle synthesis versus elongation of existing particles. Though
rubber is known to be produced by only one enzyme, extracts of latex
host numerous small molecular weight proteins with unknown function. The
proteins possibly serve as cofactors, as the synthetic rate decreases
with complete removal.
Production
Rubber
is generally cultivated in large plantations. The image shows a coconut
shell used in collecting latex, in plantations in Kerala, India.
Close to 28 million tons of rubber were produced in 2013, of which
approximately 44% was natural. Since the bulk is synthetic, which is
derived from petroleum, the price of natural rubber is determined, to a
large extent, by the prevailing global price of crude oil. Asia was the main source of natural rubber, accounting for about 94% of output in 2005. The three largest producers, Thailand, Indonesia (2.4 million tons)
and Malaysia, together account for around 72% of all natural rubber
production. Natural rubber is not cultivated widely in its native
continent of South America due to the existence of South American leaf blight, and other natural predators.
Cultivation
Rubber
latex is extracted from rubber trees. The economic life period of
rubber trees in plantations is around 32 years — up to 7 years of
immature phase and about 25 years of productive phase.
The soil requirement is well-drained, weathered soil consisting of laterite, lateritic types, sedimentary types, nonlateritic red or alluvial soils.
The climatic conditions for optimum growth of rubber trees are:
- Rainfall of around 250 centimeters (98 in) evenly distributed without any marked dry season and with at least 100 rainy days per year
- Temperature range of about 20 to 34 °C, with a monthly mean of 25 to 28 °C
- Atmospheric humidity of around 80%
- About 2000 hours sunshine per year at the rate of six hours per day throughout the year
- Absence of strong winds
Many high-yielding clones have been developed for commercial
planting. These clones yield more than 2,000 kg of dry rubber per
hectare per year, under ideal conditions.
Collection
A woman in Sri Lanka harvesting rubber, circa 1920
In places such as Kerala and Sri Lanka where coconuts are in
abundance, the half shell of coconut was used as the latex collection
container. Glazed pottery or aluminium or plastic cups became more
common in Kerala and other countries. The cups are supported by a wire
that encircles the tree. This wire incorporates a spring so it can
stretch as the tree grows. The latex is led into the cup by a galvanised
"spout" knocked into the bark. Tapping normally takes place early in
the morning, when the internal pressure of the tree is highest. A good
tapper can tap a tree every 20 seconds on a standard half-spiral system,
and a common daily "task" size is between 450 and 650 trees. Trees are
usually tapped on alternate or third days, although many variations in
timing, length and number of cuts are used. "Tappers would make a slash
in the bark with a small hatchet. These slanting cuts allowed latex to
flow from ducts located on the exterior or the inner layer of bark
(cambium) of the tree. Since the cambium controls the growth of the
tree, growth stops if it is cut. Thus, rubber tapping demanded accuracy,
so that the incisions would not be too many given the size of the tree,
or too deep, which could stunt its growth or kill it."
It is usual to tap a pannel at least twice, sometimes three
times, during the tree's life. The economic life of the tree depends on
how well the tapping is carried out, as the critical factor is bark
consumption. A standard in Malaysia for alternate daily tapping is 25 cm
(vertical) bark consumption per year. The latex-containing tubes in the
bark ascend in a spiral to the right. For this reason, tapping cuts
usually ascend to the left to cut more tubes.
The trees drip latex for about four hours, stopping as latex
coagulates naturally on the tapping cut, thus blocking the latex tubes
in the bark. Tappers usually rest and have a meal after finishing their
tapping work, then start collecting the liquid "field latex" at about
midday.
Field coagula
Mixed field coagula.
Smallholder's lump at a remilling factory
The four types of field coagula are "cuplump", "treelace",
"smallholders' lump" and "earth scrap". Each has significantly different
properties.
Some trees continue to drip after the collection leading to a small
amount of "cup lump" that is collected at the next tapping. The latex
that coagulates on the cut is also collected as "tree lace". Tree lace
and cup lump together account for 10–20% of the dry rubber produced.
Latex that drips onto the ground, "earth scrap", is also collected
periodically for processing of low-grade product.
Cup lump
Cup
lump is the coagulated material found in the collection cup when the
tapper next visits the tree to tap it again. It arises from latex
clinging to the walls of the cup after the latex was last poured into
the bucket, and from late-dripping latex exuded before the
latex-carrying vessels of the tree become blocked. It is of higher
purity and of greater value than the other three types.
Tree lace
Tree
lace is the coagulum strip that the tapper peels off the previous cut
before making a new cut. It usually has higher copper and manganese
contents than cup lump. Both copper and manganese are pro-oxidants and
can damage the physical properties of the dry rubber.
Smallholders' lump
Smallholders'
lump is produced by smallholders who collect rubber from trees far from
the nearest factory. Many Indonesian smallholders, who farm paddies in
remote areas, tap dispersed trees on their way to work in the paddy
fields and collect the latex (or the coagulated latex) on their way
home. As it is often impossible to preserve the latex sufficiently to
get it to a factory that processes latex in time for it to be used to
make high quality products, and as the latex would anyway have
coagulated by the time it reached the factory, the smallholder will
coagulate it by any means available, in any container available. Some
smallholders use small containers, buckets etc., but often the latex is
coagulated in holes in the ground, which are usually lined with plastic
sheeting. Acidic materials and fermented fruit juices are used to
coagulate the latex — a form of assisted biological coagulation. Little
care is taken to exclude twigs, leaves, and even bark from the lumps
that are formed, which may also include tree lace.
Earth scrap
Earth
scrap is material that gathers around the base of the tree. It arises
from latex overflowing from the cut and running down the bark, from rain
flooding a collection cup containing latex, and from spillage from
tappers' buckets during collection. It contains soil and other
contaminants, and has variable rubber content, depending on the amount
of contaminants. Earth scrap is collected by field workers two or three
times a year and may be cleaned in a scrap-washer to recover the rubber,
or sold to a contractor who cleans it and recovers the rubber. It is of
low quality.
Processing
Removing coagulum from coagulating troughs.
Latex coagulates in the cups if kept for long and must be collected
before this happens. The collected latex, "field latex", is transferred
into coagulation tanks for the preparation of dry rubber or transferred
into air-tight containers with sieving for ammoniation. Ammoniation
preserves the latex in a colloidal state for longer periods of time.
Latex is generally processed into either latex concentrate for
manufacture of dipped goods or coagulated under controlled, clean
conditions using formic acid. The coagulated latex can then be processed
into the higher-grade, technically specified block rubbers such as SVR
3L or SVR CV or used to produce Ribbed Smoke Sheet grades.
Naturally coagulated rubber (cup lump) is used in the manufacture
of TSR10 and TSR20 grade rubbers. Processing for these grades is a size
reduction and cleaning process to remove contamination and prepare the
material for the final stage of drying.
The dried material is then baled and palletized for storage and shipment.
Vulcanized rubber
Torn latex rubber dry suit wrist seal
Natural rubber is often vulcanized, a process by which the rubber is heated and sulfur, peroxide or bisphenol are added to improve resistance and elasticity and to prevent it from perishing. Carbon black
is often used as an additive to rubber to improve its strength,
especially in vehicle tires, which account for about 70% (~9 million
tons) of carbon black production.
Transportation
Natural
rubber latex is shipped from factories in south-west Asia, South
America, and West and Center Africa to destinations around the world. As
the cost of natural rubber has risen significantly and rubber products
are dense, the shipping methods offering the lowest cost per unit weight
are preferred. Depending on destination, warehouse availability, and
transportation conditions, some methods are preferred by certain buyers.
In international trade, latex rubber is mostly shipped in 20-foot ocean
containers. Inside the container, smaller containers are used to store
the latex.
Uses
Compression molded (cured) rubber boots before the flashes are removed
Uncured rubber is used for cements; for adhesive, insulating, and friction tapes; and for crepe rubber used in insulating blankets and footwear. Vulcanized rubber
has many more applications. Resistance to abrasion makes softer kinds
of rubber valuable for the treads of vehicle tires and conveyor belts,
and makes hard rubber valuable for pump housings and piping used in the
handling of abrasive sludge.
The flexibility of rubber is appealing in hoses, tires and
rollers for devices ranging from domestic clothes wringers to printing
presses; its elasticity makes it suitable for various kinds of shock
absorbers and for specialized machinery mountings designed to reduce
vibration. Its relative gas impermeability makes it useful in the
manufacture of articles such as air hoses, balloons, balls and cushions.
The resistance of rubber to water and to the action of most fluid
chemicals has led to its use in rainwear, diving gear, and chemical and
medicinal tubing, and as a lining for storage tanks, processing
equipment and railroad tank cars. Because of their electrical
resistance, soft rubber goods are used as insulation and for protective
gloves, shoes and blankets; hard rubber is used for articles such as
telephone housings, parts for radio sets, meters and other electrical
instruments. The coefficient of friction of rubber, which is high on dry
surfaces and low on wet surfaces, leads to its use for
power-transmission belting and for water-lubricated bearings in
deep-well pumps. Indian rubber balls or lacrosse balls are made of rubber.
Around 25 million tonnes of rubber are produced each year, of which 30 percent is natural.
The remainder is synthetic rubber derived from petrochemical sources.
The top end of latex production results in latex products such as
surgeons' gloves, condoms, balloons and other relatively high-value
products. The mid-range which comes from the technically specified
natural rubber materials ends up largely in tires but also in conveyor
belts, marine products, windshield wipers and miscellaneous goods.
Natural rubber offers good elasticity, while synthetic materials tend to
offer better resistance to environmental factors such as oils,
temperature, chemicals and ultraviolet light. "Cured rubber" is rubber
that has been compounded and subjected to the vulcanisation process to
create cross-links within the rubber matrix.
Allergic reactions
Some people have a serious latex allergy, and exposure to natural latex rubber products such as latex gloves can cause anaphylactic shock. The antigenic proteins found in Hevea latex may be deliberately reduced (though not eliminated) through processing.
Latex from non-Hevea sources, such as Guayule, can be used without allergic reaction by persons with an allergy to Hevea latex.
Some allergic reactions are not to the latex itself, but from
residues of chemicals used to accelerate the cross-linking process.
Although this may be confused with an allergy to latex, it is distinct
from it, typically taking the form of Type IV hypersensitivity in the presence of traces of specific processing chemicals.
Microbial degradation
Natural rubber is susceptible to degradation by a wide range of bacteria.
The bacteria Streptomyces coelicolor, Pseudomonas citronellolis, and Nocardia spp. are capable of degrading vulcanized natural rubber.