Equipment
for running size-exclusion chromatography. The buffer is pumped through
the column (right) by a computer-controlled device
| |
| Acronym | SEC |
|---|---|
| Classification | Chromatography |
| Analytes | macromolecules synthetic polymers biomolecules |
| Manufacturers | GE, Bio-Rad, Knauer, emp Biotech |
| Other techniques | |
| Related | High-performance liquid chromatography Aqueous normal-phase chromatography Ion exchange chromatography Micellar liquid chromatography |
Size-exclusion chromatography (SEC), also known as molecular sieve chromatography, is a chromatographic method in which molecules in solution are separated by their size, and in some cases molecular weight. It is usually applied to large molecules or macromolecular complexes such as proteins and industrial polymers. Typically, when an aqueous solution is used to transport the sample through the column, the technique is known as gel-filtration chromatography, versus the name gel permeation chromatography, which is used when an organic solvent is used as a mobile phase. The chromatography column is packed with fine, porous beads which are composed of dextran polymers (Sephadex), agarose (Sepharose), or polyacrylamide (Sephacryl or BioGel P). The pore sizes of these beads are used to estimate the dimensions of macromolecules. SEC is a widely used polymer characterization method because of its ability to provide good molar mass distribution (Mw) results for polymers.
Applications
The main application of gel-filtration chromatography is the fractionation
of proteins and other water-soluble polymers, while gel permeation
chromatography is used to analyze the molecular weight distribution of
organic-soluble polymers. Either technique should not be confused with gel electrophoresis,
where an electric field is used to "pull" or "push" molecules through
the gel depending on their electrical charges. The amount of time a
solute remains within a pore is dependent on the size of the pore.
Larger solutes will have access to a smaller volume and vice versa.
Therefore, a smaller solute will remain within the pore for a longer
period of time compared to a larger solute.
Another use of size exclusion chromatography is to examine the stability and characteristics of natural organic matter in water.
In this method, Margit B. Muller, Daniel Schmitt, and Fritz H. Frimmel
tested water sources from different places in the world to determine how
stable the natural organic matter is over a period of time.
Even though, size exclusion chromatography is widely utilized to study
natural organic material, there are limitations. One of these
limitations include that there is no standard molecular weight marker; thus, there is nothing to compare the results back to. If precise molecular weight is required, other methods should be used.
Advantages
The
advantages of this method include good separation of large molecules
from the small molecules with a minimal volume of eluate,
and that various solutions can be applied without interfering with the
filtration process, all while preserving the biological activity of the
particles to separate. The technique is generally combined with others
that further separate molecules by other characteristics, such as
acidity, basicity, charge, and affinity for certain compounds. With size
exclusion chromatography, there are short and well-defined separation
times and narrow bands, which lead to good sensitivity. There is also no
sample loss because solutes do not interact with the stationary phase.
The other advantage to this experimental method is that in
certain cases, it is feasible to determine the approximate molecular
weight of a compound. The shape and size of the compound (eluent)
determine how the compound interacts with the gel (stationary phase). To
determine approximate molecular weight, the elution volumes of
compounds with their corresponding molecular weights are obtained and
then a plot of “Kav” vs “log(Mw)” is made, where and
Mw is the molecular mass. This plot acts as a calibration curve, which
is used to approximate the desired compound’s molecular weight. The Ve
component represents the volume at which the intermediate molecules
elute such as molecules that have partial access to the beads of the
column. In addition, Vt is the sum of the total volume between the beads and the volume within the beads. The Vo component represents the volume at which the larger molecules elute, which elute in the beginning.
Disadvantages are, for example, that only a limited number of bands can
be accommodated because the time scale of the chromatogram is short,
and, in general, there must be a 10% difference in molecular mass to
have a good resolution.
Discovery
The technique was invented by Grant Henry Lathe and Colin R Ruthven, working at Queen Charlotte’s Hospital, London. They later received the John Scott Award for this invention. While Lathe and Ruthven used starch gels as the matrix, Jerker Porath and Per Flodin later introduced dextran gels;
other gels with size fractionation properties include agarose and
polyacrylamide. A short review of these developments has appeared.
There were also attempts to fractionate synthetic high polymers; however, it was not until 1964, when J. C. Moore of the Dow Chemical Company published his work on the preparation of gel permeation chromatography (GPC) columns based on cross-linked polystyrene with controlled pore size,
that a rapid increase of research activity in this field began. It was
recognized almost immediately that with proper calibration, GPC was
capable to provide molar mass and molar mass distribution
information for synthetic polymers. Because the latter information was
difficult to obtain by other methods, GPC came rapidly into extensive
use.
Theory and method
SEC is used primarily for the analysis of large molecules such as
proteins or polymers. SEC works by trapping smaller molecules in the
pores of the adsorbent
("stationary phase"). This process is usually performed within a
column, which typically consists of a hollow tube tightly packed with
micron-scale polymer beads containing pores of different sizes. These
pores may be depressions on the surface or channels through the bead. As
the solution travels down the column some particles enter into the
pores. Larger particles cannot enter into as many pores. The larger the
particles, the faster the elution. The larger molecules simply pass by
the pores because those molecules are too large to enter the pores.
Larger molecules therefore flow through the column more quickly than
smaller molecules, that is, the smaller the molecule, the longer the
retention time.
One requirement for SEC is that the analyte does not interact
with the surface of the stationary phases, with differences in elution
time between analytes ideally being based solely on the solute volume
the analytes can enter, rather than chemical or electrostatic
interactions with the stationary phases. Thus, a small molecule that can
penetrate every region of the stationary phase pore system can enter a
total volume equal to the sum of the entire pore volume and the
interparticle volume. This small molecule elutes late (after the
molecule has penetrated all of the pore- and interparticle
volume—approximately 80% of the column volume). At the other extreme, a
very large molecule that cannot penetrate any the smaller pores can
enter only the interparticle volume (~35% of the column volume) and
elutes earlier when this volume of mobile phase has passed through the
column. The underlying principle of SEC is that particles of different
sizes elute
(filter) through a stationary phase at different rates. This results in
the separation of a solution of particles based on size. Provided that
all the particles are loaded simultaneously or near-simultaneously,
particles of the same size should elute together.
However, as there are various measures of the size of a macromolecule (for instance, the radius of gyration
and the hydrodynamic radius), a fundamental problem in the theory of
SEC has been the choice of a proper molecular size parameter by which
molecules of different kinds are separated. Experimentally, Benoit and
co-workers found an excellent correlation between elution volume and a
dynamically based molecular size, the hydrodynamic volume, for several different chain architecture and chemical compositions. The observed correlation based on the hydrodynamic volume became accepted as the basis of universal SEC calibration.
Still, the use of the hydrodynamic volume, a size based on
dynamical properties, in the interpretation of SEC data is not fully
understood.
This is because SEC is typically run under low flow rate conditions
where hydrodynamic factor should have little effect on the separation.
In fact, both theory and computer simulations assume a thermodynamic
separation principle: the separation process is determined by the
equilibrium distribution (partitioning) of solute macromolecules between
two phases — a dilute bulk solution phase located at the interstitial
space and confined solution phases within the pores of column packing
material. Based on this theory, it has been shown that the relevant size
parameter to the partitioning of polymers in pores is the mean span
dimension (mean maximal projection onto a line).
Although this issue has not been fully resolved, it is likely that the
mean span dimension and the hydrodynamic volume are strongly correlated.
A size exclusion column.
Each size exclusion column has a range of molecular weights that can
be separated. The exclusion limit defines the molecular weight at the
upper end of the column 'working' range and is where molecules are too
large to get trapped in the stationary phase. The lower end of the range
is defined by the permeation limit, which defines the molecular weight
of a molecule that is small enough to penetrate all pores of the
stationary phase. All molecules below this molecular mass are so small
that they elute as a single band.
The filtered solution that is collected at the end is known as the eluate. The void volume includes any particles too large to enter the medium, and the solvent volume is known as the column volume.
Factors affecting filtration
An illustration of the theory behind size exclusion chromatography
In real-life situations, particles in solution do not have a fixed
size, resulting in the probability that a particle that would otherwise
be hampered by a pore passing right by it. Also, the stationary-phase
particles are not ideally defined; both particles and pores may vary in
size. Elution curves, therefore, resemble Gaussian distributions.
The stationary phase may also interact in undesirable ways with a
particle and influence retention times, though great care is taken by
column manufacturers to use stationary phases that are inert and
minimize this issue.
Like other forms of chromatography, increasing the column length
enhances resolution, and increasing the column diameter increases column
capacity. Proper column packing is important for maximum resolution: An
over-packed column can collapse the pores in the beads, resulting in a
loss of resolution. An under-packed column can reduce the relative
surface area of the stationary phase accessible to smaller species,
resulting in those species spending less time trapped in pores. Unlike
affinity chromatography techniques, a solvent head at the top of the
column can drastically diminish resolution as the sample diffuses prior
to loading, broadening the downstream elution.
Analysis
In
simple manual columns, the eluent is collected in constant volumes,
known as fractions. The more similar the particles are in size the more
likely they are in the same fraction and not detected separately. More
advanced columns overcome this problem by constantly monitoring the
eluent.
Standardization of a size exclusion column.
The collected fractions are often examined by spectroscopic techniques to determine the concentration of the particles eluted. Common spectroscopy detection techniques are refractive index
(RI) and ultraviolet (UV). When eluting spectroscopically similar
species (such as during biological purification), other techniques may
be necessary to identify the contents of each fraction. It is also
possible to analyze the eluent flow continuously with RI, LALLS, Multi-Angle Laser Light Scattering MALS, UV, and/or viscosity measurements.
SEC Chromatogram of a biological sample.
The elution volume (Ve) decreases roughly linear with the logarithm of the molecular hydrodynamic volume.
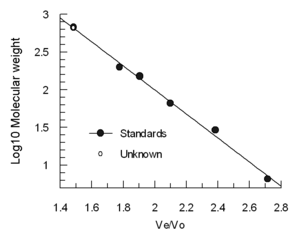
Columns are often calibrated using 4-5 standard samples (e.g., folded
proteins of known molecular weight), and a sample containing a very
large molecule such as thyroglobulin to determine the void volume.
(Blue dextran is not recommended for Vo determination because it is
heterogeneous and may give variable results) The elution volumes of the
standards are divided by the elution volume of the thyroglobulin (Ve/Vo)
and plotted against the log of the standards' molecular weights.
Applications
Biochemical applications
In
general, SEC is considered a low resolution chromatography as it does
not discern similar species very well, and is therefore often reserved
for the final step of a purification. The technique can determine the quaternary structure of purified proteins that have slow exchange times, since it can be carried out under native solution conditions, preserving macromolecular interactions. SEC can also assay protein tertiary structure,
as it measures the hydrodynamic volume (not molecular weight), allowing
folded and unfolded versions of the same protein to be distinguished.
For example, the apparent hydrodynamic radius
of a typical protein domain might be 14 Å and 36 Å for the folded and
unfolded forms, respectively. SEC allows the separation of these two
forms, as the folded form elutes much later due to its smaller size.
Polymer synthesis
SEC can be used as a measure of both the size and the polydispersity of a synthesised polymer,
that is, the ability to find the distribution of the sizes of polymer
molecules. If standards of a known size are run previously, then a calibration curve can be created to determine the sizes of polymer molecules of interest in the solvent chosen for analysis (often THF). In alternative fashion, techniques such as light scattering and/or viscometry
can be used online with SEC to yield absolute molecular weights that do
not rely on calibration with standards of known molecular weight. Due
to the difference in size of two polymers with identical molecular
weights, the absolute determination methods are, in general, more
desirable. A typical SEC system can quickly (in about half an hour) give
polymer chemists information on the size and polydispersity of the
sample. The preparative SEC can be used for polymer fractionation on an analytical scale.
Drawback
In
SEC, mass is not measured so much as the hydrodynamic volume of the
polymer molecules, that is, how much space a particular polymer molecule
takes up when it is in solution. However, the approximate molecular
weight can be calculated from SEC data because the exact relationship
between molecular weight and hydrodynamic volume for polystyrene can be
found. For this, polystyrene is used as a standard. But the relationship
between hydrodynamic volume and molecular weight is not the same for
all polymers, so only an approximate measurement can be obtained.
Another drawback is the possibility of interaction between the
stationary phase and the analyte. Any interaction leads to a later
elution time and thus mimics a smaller analyte size.
When performing this method, the bands of the eluting molecules
may be broadened. This can occur by turbulence caused by the flow of the
mobile phase molecules passing through the molecules of the stationary
phase. In addition, molecular thermal diffusion and friction
between the molecules of the glass walls and the molecules of the
eluent contribute to the broadening of the bands. Besides broadening,
the bands also overlap with each other. As a result, the eluent usually
gets considerably diluted. A few precautions can be taken to prevent the
likelihood of the bands broadening. For instance, one can apply the
sample in a narrow, highly concentrated band on the top of the column.
The more concentrated the eluent is, the more efficient the procedure
would be. However, it is not always possible to concentrate the eluent,
which can be considered as one more disadvantage.
Absolute size-exclusion chromatography
Absolute size-exclusion chromatography (ASEC) is a technique that couples a dynamic light scattering
(DLS) instrument to a size exclusion chromatography system for absolute
size measurements of proteins and macromolecules as they elute from the
chromatography system.
The definition of “absolute” in this case is that calibration is
not required to obtain hydrodynamic size, often referred to as
hydrodynamic diameter (DH in units of nm). The sizes of the
macromolecules are measured as they elute into the flow cell of the DLS
instrument from the size exclusion column set. It should be noted that
the hydrodynamic size of the molecules or particles are measured and not
their molecular weights. For proteins a Mark-Houwink type of
calculation can be used to estimate the molecular weight from the
hydrodynamic size.
A major advantage of DLS coupled with SEC is the ability to obtain enhanced DLS resolution.
Batch DLS is quick and simple and provides a direct measure of the
average size, but the baseline resolution of DLS is 3 to 1 in diameter.
Using SEC, the proteins and protein oligomers are separated, allowing
oligomeric resolution. Aggregation studies can also be done using ASEC.
Though the aggregate concentration may not be calculated, the size of
the aggregate can be measured, only limited by the maximum size eluting
from the SEC columns.
Limitations of ASEC include flow-rate, concentration, and
precision. Because a correlation function requires anywhere from 3–7
seconds to properly build, a limited number of data points can be
collected across the peak.