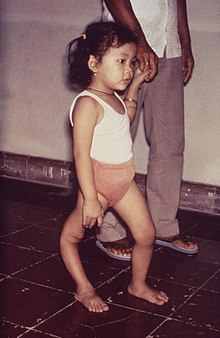| Polio | |
|---|---|
| Other names | Poliomyelitis, infantile paralysis, Heine-Medin disease |
 | |
| A man with a smaller right leg due to poliomyelitis | |
| Pronunciation | |
| Specialty | Neurology, Infectious disease |
| Symptoms | Muscle weakness resulting in an inability to move |
| Complications | Post-polio syndrome |
| Usual onset | Few hours to days |
| Causes | Poliovirus spread by fecal-oral route |
| Diagnostic method | Finding the virus in the feces or antibodies in the blood |
| Prevention | Polio vaccine |
| Treatment | Supportive care |
| Frequency | 136 people (2018) |
Poliomyelitis, commonly shortened to polio, is an infectious disease caused by the poliovirus. In about 0.5 percent of cases, it moves from the gut to affect the central nervous system and there is muscle weakness resulting in a flaccid paralysis. This can occur over a few hours to a few days. The weakness most often involves the legs, but may less commonly involve the muscles of the head, neck and diaphragm. Many people fully recover. In those with muscle weakness, about 2 to 5 percent of children and 15 to 30 percent of adults die. Up to 70 percent of those infected have no symptoms. Another 25 percent of people have minor symptoms such as fever and a sore throat, and up to 5 percent have headache, neck stiffness and pains in the arms and legs. These people are usually back to normal within one or two weeks. Years after recovery, post-polio syndrome may occur, with a slow development of muscle weakness similar to that which the person had during the initial infection.
Poliovirus is usually spread from person to person through infected fecal matter entering the mouth. It may also be spread by food or water containing human feces and less commonly from infected saliva. Those who are infected may spread the disease for up to six weeks even if no symptoms are present. The disease may be diagnosed by finding the virus in the feces or detecting antibodies against it in the blood. The disease occurs naturally only in humans.
The disease is preventable with the polio vaccine; however, multiple doses are required for it to be effective. The US Centers for Disease Control and Prevention recommends polio vaccination boosters for travelers and those who live in countries where the disease is endemic. Once infected there is no specific treatment. In 2018, there were 33 cases of wild polio and 104 cases of vaccine-derived polio. This is down from 350,000 wild cases in 1988. In 2018, the wild disease was spread between people only in Afghanistan and Pakistan. In 2019, there were 175 cases of wild polio and 364 cases of vaccine-derived polio.
Poliomyelitis has existed for thousands of years, with depictions of the disease in ancient art. The disease was first recognized as a distinct condition by the English physician Michael Underwood in 1789 and the virus that causes it was first identified in 1909 by the Austrian immunologist Karl Landsteiner. Major outbreaks started to occur in the late 19th century in Europe and the United States. In the 20th century it became one of the most worrying childhood diseases in these areas. The first polio vaccine was developed in the 1950s by Jonas Salk. Soon after, Albert Sabin developed an oral vaccine, which has become the world standard.
Signs and symptoms
| Outcome | Proportion of cases |
|---|---|
| No symptoms | 72% |
| Minor illness | 24% |
| Nonparalytic aseptic meningitis |
1–5% |
| Paralytic poliomyelitis | 0.1–0.5% |
| — Spinal polio | 79% of paralytic cases |
| — Bulbospinal polio | 19% of paralytic cases |
| — Bulbar polio | 2% of paralytic cases |
The term "poliomyelitis" is used to identify the disease caused by any of the three serotypes of poliovirus. Two basic patterns of polio infection are described: a minor illness which does not involve the central nervous system (CNS), sometimes called abortive poliomyelitis, and a major illness involving the CNS, which may be paralytic or nonparalytic. In most people with a normal immune system, a poliovirus infection is asymptomatic. Rarely, the infection produces minor symptoms; these may include upper respiratory tract infection (sore throat and fever), gastrointestinal disturbances (nausea, vomiting, abdominal pain, constipation or, rarely, diarrhea), and influenza-like illness.
The virus enters the central nervous system in about 1 percent of infections. Most patients with CNS involvement develop nonparalytic aseptic meningitis, with symptoms of headache, neck, back, abdominal and extremity pain, fever, vomiting, lethargy, and irritability. About one to five in 1000 cases progress to paralytic disease, in which the muscles become weak, floppy and poorly controlled, and, finally, completely paralyzed; this condition is known as acute flaccid paralysis. Depending on the site of paralysis, paralytic poliomyelitis is classified as spinal, bulbar, or bulbospinal. Encephalitis, an infection of the brain tissue itself, can occur in rare cases, and is usually restricted to infants. It is characterized by confusion, changes in mental status, headaches, fever, and, less commonly, seizures and spastic paralysis.
Cause
Poliomyelitis is caused by infection with a member of the genus Enterovirus known as poliovirus (PV). This group of RNA viruses colonize the gastrointestinal tract – specifically the oropharynx and the intestine. The incubation time (to the first signs and symptoms) ranges from three to 35 days, with a more common span of six to 20 days. PV infects and causes disease in humans alone. Its structure is very simple, composed of a single (+) sense RNA genome enclosed in a protein shell called a capsid. In addition to protecting the virus' genetic material, the capsid proteins enable poliovirus to infect certain types of cells. Three serotypes of poliovirus have been identified – poliovirus type 1 (PV1), type 2 (PV2), and type 3 (PV3) – each with a slightly different capsid protein. All three are extremely virulent and produce the same disease symptoms. PV1 is the most commonly encountered form, and the one most closely associated with paralysis.
Individuals who are exposed to the virus, either through infection or by immunization with polio vaccine, develop immunity. In immune individuals, IgA antibodies against poliovirus are present in the tonsils and gastrointestinal tract, and are able to block virus replication; IgG and IgM antibodies against PV can prevent the spread of the virus to motor neurons of the central nervous system. Infection or vaccination with one serotype of poliovirus does not provide immunity against the other serotypes, and full immunity requires exposure to each serotype.
A rare condition with a similar presentation, nonpoliovirus poliomyelitis, may result from infections with nonpoliovirus enteroviruses.
Transmission
Poliomyelitis is highly contagious via the fecal-oral (intestinal source) and the oral-oral (oropharyngeal source) routes. In endemic areas, wild polioviruses can infect virtually the entire human population. It is seasonal in temperate climates, with peak transmission occurring in summer and autumn. These seasonal differences are far less pronounced in tropical areas. The time between first exposure and first symptoms, known as the incubation period, is usually 6 to 20 days, with a maximum range of 3 to 35 days. Virus particles are excreted in the feces for several weeks following initial infection. The disease is transmitted primarily via the fecal-oral route, by ingesting contaminated food or water. It is occasionally transmitted via the oral-oral route, a mode especially visible in areas with good sanitation and hygiene. Polio is most infectious between 7 and 10 days before and after the appearance of symptoms, but transmission is possible as long as the virus remains in the saliva or feces.
Factors that increase the risk of polio infection or affect the severity of the disease include immune deficiency, malnutrition, physical activity immediately following the onset of paralysis, skeletal muscle injury due to injection of vaccines or therapeutic agents, and pregnancy. Although the virus can cross the maternal-fetal barrier during pregnancy, the fetus does not appear to be affected by either maternal infection or polio vaccination. Maternal antibodies also cross the placenta, providing passive immunity that protects the infant from polio infection during the first few months of life.
Pathophysiology
Poliovirus enters the body through the mouth, infecting the first cells with which it comes in contact – the pharynx and intestinal mucosa. It gains entry by binding to an immunoglobulin-like receptor, known as the poliovirus receptor or CD155, on the cell membrane. The virus then hijacks the host cell's own machinery, and begins to replicate. Poliovirus divides within gastrointestinal cells for about a week, from where it spreads to the tonsils, the intestinal lymphoid tissue including the M cells of Peyer's patches, and the deep cervical and mesenteric lymph nodes, where it multiplies abundantly. The virus is subsequently absorbed into the bloodstream.
Known as viremia, the presence of a virus in the bloodstream enables it to be widely distributed throughout the body. Poliovirus can survive and multiply within the blood and lymphatics for long periods of time, sometimes as long as 17 weeks. In a small percentage of cases, it can spread and replicate in other sites, such as brown fat, the reticuloendothelial tissues, and muscle. This sustained replication causes a major viremia, and leads to the development of minor influenza-like symptoms. Rarely, this may progress and the virus may invade the central nervous system, provoking a local inflammatory response. In most cases, this causes a self-limiting inflammation of the meninges, the layers of tissue surrounding the brain, which is known as nonparalytic aseptic meningitis. Penetration of the CNS provides no known benefit to the virus, and is quite possibly an incidental deviation of a normal gastrointestinal infection. The mechanisms by which poliovirus spreads to the CNS are poorly understood, but it appears to be primarily a chance event – largely independent of the age, gender, or socioeconomic position of the individual.
Paralytic polio
In around one percent of infections, poliovirus spreads along certain nerve fiber pathways, preferentially replicating in and destroying motor neurons within the spinal cord, brain stem, or motor cortex. This leads to the development of paralytic poliomyelitis, the various forms of which (spinal, bulbar, and bulbospinal) vary only with the amount of neuronal damage and inflammation that occurs, and the region of the CNS affected.
The destruction of neuronal cells produces lesions within the spinal ganglia; these may also occur in the reticular formation, vestibular nuclei, cerebellar vermis, and deep cerebellar nuclei. Inflammation associated with nerve cell destruction often alters the color and appearance of the gray matter in the spinal column, causing it to appear reddish and swollen. Other destructive changes associated with paralytic disease occur in the forebrain region, specifically the hypothalamus and thalamus. The molecular mechanisms by which poliovirus causes paralytic disease are poorly understood.
Early symptoms of paralytic polio include high fever, headache, stiffness in the back and neck, asymmetrical weakness of various muscles, sensitivity to touch, difficulty swallowing, muscle pain, loss of superficial and deep reflexes, paresthesia (pins and needles), irritability, constipation, or difficulty urinating. Paralysis generally develops one to ten days after early symptoms begin, progresses for two to three days, and is usually complete by the time the fever breaks.
The likelihood of developing paralytic polio increases with age, as does the extent of paralysis. In children, nonparalytic meningitis is the most likely consequence of CNS involvement, and paralysis occurs in only one in 1000 cases. In adults, paralysis occurs in one in 75 cases. In children under five years of age, paralysis of one leg is most common; in adults, extensive paralysis of the chest and abdomen also affecting all four limbs – quadriplegia – is more likely. Paralysis rates also vary depending on the serotype of the infecting poliovirus; the highest rates of paralysis (one in 200) are associated with poliovirus type 1, the lowest rates (one in 2,000) are associated with type 2.
Spinal polio
Spinal polio, the most common form of paralytic poliomyelitis, results from viral invasion of the motor neurons of the anterior horn cells, or the ventral (front) grey matter section in the spinal column, which are responsible for movement of the muscles, including those of the trunk, limbs, and the intercostal muscles. Virus invasion causes inflammation of the nerve cells, leading to damage or destruction of motor neuron ganglia. When spinal neurons die, Wallerian degeneration takes place, leading to weakness of those muscles formerly innervated by the now-dead neurons. With the destruction of nerve cells, the muscles no longer receive signals from the brain or spinal cord; without nerve stimulation, the muscles atrophy, becoming weak, floppy and poorly controlled, and finally completely paralyzed. Maximum paralysis progresses rapidly (two to four days), and usually involves fever and muscle pain. Deep tendon reflexes are also affected, and are typically absent or diminished; sensation (the ability to feel) in the paralyzed limbs, however, is not affected.
The extent of spinal paralysis depends on the region of the cord affected, which may be cervical, thoracic, or lumbar. The virus may affect muscles on both sides of the body, but more often the paralysis is asymmetrical. Any limb or combination of limbs may be affected – one leg, one arm, or both legs and both arms. Paralysis is often more severe proximally (where the limb joins the body) than distally (the fingertips and toes).
Bulbar polio
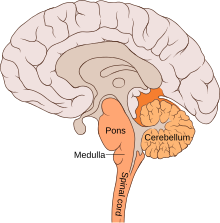
Making up about two percent of cases of paralytic polio, bulbar polio occurs when poliovirus invades and destroys nerves within the bulbar region of the brain stem. The bulbar region is a white matter pathway that connects the cerebral cortex to the brain stem. The destruction of these nerves weakens the muscles supplied by the cranial nerves, producing symptoms of encephalitis, and causes difficulty breathing, speaking and swallowing. Critical nerves affected are the glossopharyngeal nerve (which partially controls swallowing and functions in the throat, tongue movement, and taste), the vagus nerve (which sends signals to the heart, intestines, and lungs), and the accessory nerve (which controls upper neck movement). Due to the effect on swallowing, secretions of mucus may build up in the airway, causing suffocation. Other signs and symptoms include facial weakness (caused by destruction of the trigeminal nerve and facial nerve, which innervate the cheeks, tear ducts, gums, and muscles of the face, among other structures), double vision, difficulty in chewing, and abnormal respiratory rate, depth, and rhythm (which may lead to respiratory arrest). Pulmonary edema and shock are also possible and may be fatal.
Bulbospinal polio
Approximately 19 percent of all paralytic polio cases have both bulbar and spinal symptoms; this subtype is called respiratory or bulbospinal polio. Here, the virus affects the upper part of the cervical spinal cord (cervical vertebrae C3 through C5), and paralysis of the diaphragm occurs. The critical nerves affected are the phrenic nerve (which drives the diaphragm to inflate the lungs) and those that drive the muscles needed for swallowing. By destroying these nerves, this form of polio affects breathing, making it difficult or impossible for the patient to breathe without the support of a ventilator. It can lead to paralysis of the arms and legs and may also affect swallowing and heart functions.
Diagnosis
Paralytic poliomyelitis may be clinically suspected in individuals experiencing acute onset of flaccid paralysis in one or more limbs with decreased or absent tendon reflexes in the affected limbs that cannot be attributed to another apparent cause, and without sensory or cognitive loss.
A laboratory diagnosis is usually made based on recovery of poliovirus from a stool sample or a swab of the pharynx. Antibodies to poliovirus can be diagnostic, and are generally detected in the blood of infected patients early in the course of infection. Analysis of the patient's cerebrospinal fluid (CSF), which is collected by a lumbar puncture ("spinal tap"), reveals an increased number of white blood cells (primarily lymphocytes) and a mildly elevated protein level. Detection of virus in the CSF is diagnostic of paralytic polio, but rarely occurs.
If poliovirus is isolated from a patient experiencing acute flaccid paralysis, it is further tested through oligonucleotide mapping (genetic fingerprinting), or more recently by PCR amplification, to determine whether it is "wild type" (that is, the virus encountered in nature) or "vaccine type" (derived from a strain of poliovirus used to produce polio vaccine). It is important to determine the source of the virus because for each reported case of paralytic polio caused by wild poliovirus, an estimated 200 to 3,000 other contagious asymptomatic carriers exist.
Prevention
Passive immunization
In 1950, William Hammon at the University of Pittsburgh purified the gamma globulin component of the blood plasma of polio survivors. Hammon proposed the gamma globulin, which contained antibodies to poliovirus, could be used to halt poliovirus infection, prevent disease, and reduce the severity of disease in other patients who had contracted polio. The results of a large clinical trial were promising; the gamma globulin was shown to be about 80 percent effective in preventing the development of paralytic poliomyelitis. It was also shown to reduce the severity of the disease in patients who developed polio. Due to the limited supply of blood plasma gamma globulin was later deemed impractical for widespread use and the medical community focused on the development of a polio vaccine.
Vaccine
Two types of vaccine are used throughout the world to combat polio. Both types induce immunity to polio, efficiently blocking person-to-person transmission of wild poliovirus, thereby protecting both individual vaccine recipients and the wider community (so-called herd immunity).
The first candidate polio vaccine, based on one serotype of a live but attenuated (weakened) virus, was developed by the virologist Hilary Koprowski. Koprowski's prototype vaccine was given to an eight-year-old boy on 27 February 1950. Koprowski continued to work on the vaccine throughout the 1950s, leading to large-scale trials in the then Belgian Congo and the vaccination of seven million children in Poland against serotypes PV1 and PV3 between 1958 and 1960.
The second inactivated polio virus vaccine was developed in 1952 by Jonas Salk at the University of Pittsburgh, and announced to the world on 12 April 1955. The Salk vaccine, or inactivated poliovirus vaccine, is based on poliovirus grown in a type of monkey kidney tissue culture (vero cell line), which is chemically inactivated with formalin. After two doses of inactivated poliovirus vaccine (given by injection), 90 percent or more of individuals develop protective antibody to all three serotypes of poliovirus, and at least 99 percent are immune to poliovirus following three doses.
Subsequently, Albert Sabin developed another live, oral polio vaccine. It was produced by the repeated passage of the virus through nonhuman cells at temperatures. The attenuated poliovirus in the Sabin vaccine replicates very efficiently in the gut, the primary site of wild poliovirus infection and replication, but the vaccine strain is unable to replicate efficiently within nervous system tissue. A single dose of Sabin's oral polio vaccine produces immunity to all three poliovirus serotypes in about 50 percent of recipients. Three doses of live-attenuated oral vaccine produce protective antibody to all three poliovirus types in more than 95 percent of recipients. Human trials of Sabin's vaccine began in 1957, and in 1958 it was selected, in competition with the live vaccines of Koprowski and other researchers, by the US National Institutes of Health. Licensed in 1962, it rapidly became the only polio vaccine used worldwide.
Because the oral polio vaccine is inexpensive, easy to administer, and produces excellent immunity in the intestine (which helps prevent infection with wild virus in areas where it is endemic), it has been the vaccine of choice for controlling poliomyelitis in many countries. On very rare occasions (about one case per 750,000 vaccine recipients), the attenuated virus in the oral polio vaccine reverts into a form that can paralyze. In 2017, cases caused by vaccine-derived poliovirus (cVDPV) outnumbered wild poliovirus cases for the first time, due to wild polio cases hitting record lows and relaxed vaccination levels. Most industrialized countries have switched to inactivated polio vaccine, which cannot revert, either as the sole vaccine against poliomyelitis or in combination with oral polio vaccine.
Treatment
There is no cure for polio, but there are treatments. The focus of modern treatment has been on providing relief of symptoms, speeding recovery and preventing complications. Supportive measures include antibiotics to prevent infections in weakened muscles, analgesics for pain, moderate exercise and a nutritious diet. Treatment of polio often requires long-term rehabilitation, including occupational therapy, physical therapy, braces, corrective shoes and, in some cases, orthopedic surgery.
Portable ventilators may be required to support breathing. Historically, a noninvasive, negative-pressure ventilator, more commonly called an iron lung, was used to artificially maintain respiration during an acute polio infection until a person could breathe independently (generally about one to two weeks). Today, many polio survivors with permanent respiratory paralysis use modern jacket-type negative-pressure ventilators worn over the chest and abdomen.
Other historical treatments for polio include hydrotherapy, electrotherapy, massage and passive motion exercises, and surgical treatments, such as tendon lengthening and nerve grafting.
Sister Elizabeth Kenny's Kenny regimen is now the hallmark for the treatment of paralytic polio.
Prognosis
Patients with abortive polio infections recover completely. In those who develop only aseptic meningitis, the symptoms can be expected to persist for two to ten days, followed by complete recovery. In cases of spinal polio, if the affected nerve cells are completely destroyed, paralysis will be permanent; cells that are not destroyed, but lose function temporarily, may recover within four to six weeks after onset. Half the patients with spinal polio recover fully; one-quarter recover with mild disability, and the remaining quarter are left with severe disability. The degree of both acute paralysis and residual paralysis is likely to be proportional to the degree of viremia, and inversely proportional to the degree of immunity. Spinal polio is rarely fatal.
Without respiratory support, consequences of poliomyelitis with respiratory involvement include suffocation or pneumonia from aspiration of secretions. Overall, 5 to 10 percent of patients with paralytic polio die due to the paralysis of muscles used for breathing. The case fatality rate (CFR) varies by age: 2 to 5 percent of children and up to 15 to 30 percent of adults die. Bulbar polio often causes death if respiratory support is not provided; with support, its CFR ranges from 25 to 75 percent, depending on the age of the patient. When intermittent positive pressure ventilation is available, the fatalities can be reduced to 15 percent.
Recovery
Many cases of poliomyelitis result in only temporary paralysis. Generally in these cases, nerve impulses return to the paralyzed muscle within a month, and recovery is complete in six to eight months. The neurophysiological processes involved in recovery following acute paralytic poliomyelitis are quite effective; muscles are able to retain normal strength even if half the original motor neurons have been lost. Paralysis remaining after one year is likely to be permanent, although some recovery of muscle strength is possible up to 18 months after infection.
One mechanism involved in recovery is nerve terminal sprouting, in which remaining brainstem and spinal cord motor neurons develop new branches, or axonal sprouts. These sprouts can reinnervate orphaned muscle fibers that have been denervated by acute polio infection, restoring the fibers' capacity to contract and improving strength. Terminal sprouting may generate a few significantly enlarged motor neurons doing work previously performed by as many as four or five units: a single motor neuron that once controlled 200 muscle cells might control 800 to 1000 cells. Other mechanisms that occur during the rehabilitation phase, and contribute to muscle strength restoration, include myofiber hypertrophy – enlargement of muscle fibers through exercise and activity – and transformation of type II muscle fibers to type I muscle fibers.
In addition to these physiological processes, the body can compensate for residual paralysis in other ways. Weaker muscles can be used at a higher than usual intensity relative to the muscle's maximal capacity, little-used muscles can be developed, and ligaments can enable stability and mobility.
Complications
Residual complications of paralytic polio often occur following the initial recovery process. Muscle paresis and paralysis can sometimes result in skeletal deformities, tightening of the joints, and movement disability. Once the muscles in the limb become flaccid, they may interfere with the function of other muscles. A typical manifestation of this problem is equinus foot (similar to club foot). This deformity develops when the muscles that pull the toes downward are working, but those that pull it upward are not, and the foot naturally tends to drop toward the ground. If the problem is left untreated, the Achilles tendons at the back of the foot retract and the foot cannot take on a normal position. Polio victims that develop equinus foot cannot walk properly because they cannot put their heel on the ground. A similar situation can develop if the arms become paralyzed. In some cases the growth of an affected leg is slowed by polio, while the other leg continues to grow normally. The result is that one leg is shorter than the other and the person limps and leans to one side, in turn leading to deformities of the spine (such as scoliosis). Osteoporosis and increased likelihood of bone fractures may occur. An intervention to prevent or lessen length disparity can be to perform an epiphysiodesis on the distal femoral and proximal tibial/fibular condyles, so that limb's growth is artificially stunted, and by the time of epiphyseal (growth) plate closure, the legs are more equal in length. Alternatively, a person can be fitted with custom made footwear which corrects the difference in leg lengths. Other surgery to re-balance muscular agonist/antagonist imbalances may also be helpful. Extended use of braces or wheelchairs may cause compression neuropathy, as well as a loss of proper function of the veins in the legs, due to pooling of blood in paralyzed lower limbs. Complications from prolonged immobility involving the lungs, kidneys and heart include pulmonary edema, aspiration pneumonia, urinary tract infections, kidney stones, paralytic ileus, myocarditis and cor pulmonale.
Post-polio syndrome
Between 25 percent and 50 percent of individuals who have recovered from paralytic polio in childhood can develop additional symptoms decades after recovering from the acute infection, notably new muscle weakness and extreme fatigue. This condition is known as post-polio syndrome (PPS) or post-polio sequelae. The symptoms of PPS are thought to involve a failure of the oversized motor units created during the recovery phase of the paralytic disease. Contributing factors that increase the risk of PPS include aging with loss of neuron units, the presence of a permanent residual impairment after recovery from the acute illness, and both overuse and disuse of neurons. PPS is a slow, progressive disease, and there is no specific treatment for it. Post-polio syndrome is not an infectious process, and persons experiencing the syndrome do not shed poliovirus.
Epidemiology
| Country | Wild cases |
Circulating vaccine- derived cases |
Transmission status |
Type |
|---|---|---|---|---|
| 146 | 22 | endemic | WPV1 cVDPV2 | |
| 29 | 0 | endemic | WPV1 | |
| 0 | 129 | cVDPV only | cVDPV2 | |
| 0 | 86 | cVDPV only | cVDPV2 | |
| 0 | 19 | cVDPV only | cVDPV2 | |
| 0 | 18 | cVDPV only | cVDPV2 | |
| 0 | 18 | cVDPV only | cVDPV2 | |
| 0 | 15 | cVDPV only | cVDPV1 cVDPV2 | |
| 0 | 12 | cVDPV only | cVDPV2 | |
| 0 | 9 | cVDPV only | cVDPV2 | |
| 0 | 8 | cVDPV only | cVDPV2 | |
| 0 | 8 | cVDPV only | cVDPV2 | |
| 0 | 6 | cVDPV only | cVDPV1 | |
| 0 | 3 | cVDPV only | cVDPV2 | |
| 0 | 3 | cVDPV only | cVDPV1 | |
| 0 | 2 | cVDPV only | cVDPV2 | |
| 0 | 1 | cVDPV only | cVDPV2 | |
| 0 | 1 | cVDPV only | cVDPV2 | |
| 0 | 1 | cVDPV only | cVDPV2 | |
| 0 | 3 | cVDPV only | cVDPV1 | |
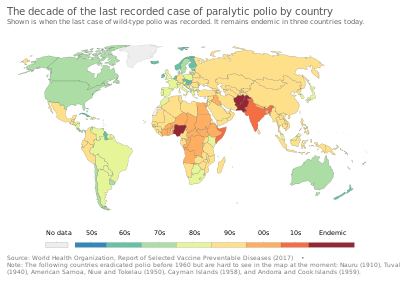
Following the widespread use of poliovirus vaccine in the mid-1950s, new cases of poliomyelitis declined dramatically in many industrialized countries. A global effort to eradicate polio began in 1988, led by the World Health Organization, UNICEF, and The Rotary Foundation. These efforts have reduced the number of cases diagnosed each year by 99.9 percent; from an estimated 350,000 cases in 1988 to a low of 483 cases in 2001, after which it remained at a level of about 1,000–2000 cases per year for a number of years.
In April 2012, the World Health Assembly declared that the failure to completely eradicate polio would be a programmatic emergency for global public health, and that it "must not happen."
In 2015, polio was believed to remain naturally spreading in only two countries, Pakistan and Afghanistan, although it continued to cause outbreaks in other nearby countries due to hidden or reestablished transmission.
In 2015, cases decreased to 98 and further decreased in 2016 to 37 wild cases and 5 circulating vaccine-derived cases, but increased in 2019 to 175 wild cases and 365 circulating vaccine-derived cases. Polio is one of only two diseases currently the subject of a global eradication program, the other being Guinea worm disease. So far, the only diseases completely eradicated by humankind are smallpox, declared so in 1980, and rinderpest, likewise in 2011.
A concern is the presence of circulating vaccine-derived polioviruses. The oral polio vaccine is not perfect: while the genetic characteristics are carefully balanced to maximize efficacy and minimize virulence, it is possible for the polio virus in the oral vaccine to mutate. As a result, persons given the oral polio vaccine can acquire acute or chronic infections; or can transmit (circulate) mutated virus to other people. Circulating vaccine-derived poliovirus cases have exceeded wild-type cases, making it desirable to discontinue use of the oral polio vaccine as soon as safely possible.
Afghanistan and Pakistan
The last remaining region with wild polio cases are the South Asian countries Afghanistan and Pakistan. Both major sides of the Afghan civil war support polio vaccination, but after declining rapidly, polio rates are increasing in Afghanistan, with 19 cases in 2015, 13 in 2016, 14 in 2017, 21 in 2018, and 29 in 2019 out of a population of about 35 million.
In Pakistan, there were 53 cases in 2015 (out of a population of about 200 million) – the highest number for any country, with 20 in 2016, 8 in 2017 12 in 2018, and 146 in 2019. Cases dropped by 97 percent from 2014 to 2018; reasons include 440 million dirham support from the United Arab Emirates to vaccinate more than ten million children, changes in the military situation, and arrests of some of those who attacked polio workers.
In Pakistan, the CIA ran a fake vaccination clinic in an attempt to locate Osama bin Laden. As a consequence, there were attacks and deaths among vaccination workers.
Several Islamist preachers and militant groups, including some factions of the Taliban, view vaccination as a plot to kill or sterilize Muslims. 66 vaccinators were killed in 2013 and 2014. This is part of the reason Pakistan and Afghanistan are the only countries where polio remained endemic as of 2015.
Americas
The Americas were declared polio-free in 1994. The last known case was a boy in Peru in 1991.
Western Pacific
In 2000, polio was declared to have been officially eliminated in 37 Western Pacific countries, including China and Australia.
Despite eradication ten years earlier, an outbreak was confirmed in China in September 2011, involving a strain common in Pakistan.
Europe
Europe was declared polio-free in 2002. On 1 September 2015, WHO confirmed two cases of circulating vaccine-derived poliovirus type 1 in Ukraine.
Southeast Asia
The last case of polio in the region was in India (part of the WHO's South-East Asia Region) in January 2011. Since January 2011, there have been no reported cases of the wild polio infections in India, and in February 2012 the country was taken off the WHO list of polio endemic countries.
On 27 March 2014, the WHO announced the eradication of poliomyelitis in the South-East Asia Region, which includes eleven countries: Bangladesh, Bhutan, North Korea, India, Indonesia, Maldives, Myanmar, Nepal, Sri Lanka, Thailand and Timor-Leste. With the addition of this region, 80 per cent of the world population was considered to be living in polio-free regions.
However, in September 2019, the Department of Health of the Philippines declared a polio outbreak in the country after a 3-year-old girl was found with the disease on the 14th.
In December 2019, acute poliomyelitis was confirmed in a 3-month-old infant in Tuaran, a town in Sabah state, Borneo, Malaysia. It was the first confirmed case in Malaysia since 1992, and Malaysia had been declared polio-free in 2000. The child reportedly had a fever and muscle weakness, and although in stable condition, required assistance to breathe. Testing of the virus indicated that it was related to the strain that had appeared in the Philippines. Local officials said the strain originated from a weakened virus used in an oral vaccine that was then excreted in feces and spread into the unvaccinated population through unsanitary conditions. It was reported that 23 of 199 children in the local community had not received the polio vaccine.
Middle East
In Syria difficulties in executing immunization programs in the ongoing civil war led to a return of polio, probably in 2012, acknowledged by the WHO in 2013. 15 cases were confirmed among children in Syria between October and November 2013 in Deir Ezzor. Later, two more cases, one each in rural Damascus and Aleppo, were identified. It was the first outbreak in Syria since 1999. Doctors and international public health agencies report more than 90 cases of polio in Syria, with fears of contagion in rebel areas from lack of sanitation and safe-water services. In May 2014, the World Health Organization declared polio's renewed spread a world health emergency.
A vaccination campaign in Syria operated literally under fire and led to the deaths of several vaccinators, but returned vaccination coverage to pre-war levels.
Another epidemic of polio was confirmed in 2017 in eastern Syria, probably resulting from a mutated form of the virus spreading through contaminated water.
Africa
In 2003 in northern Nigeria – a country which at that time was considered provisionally polio free – a fatwa was issued declaring that the polio vaccine was designed to render children sterile. Subsequently, polio reappeared in Nigeria and spread from there to several other countries. In 2013, nine health workers administering polio vaccine were targeted and killed by gunmen on motorcycles in Kano, but this was the only attack. Local traditional and religious leaders and polio survivors worked to revive the campaign, and Nigeria was removed from the polio-endemic list in September 2015 after more than a year without any cases, only to be restored to the list in 2016 when two cases were detected.
In 2013 the Center for Disease Control received reports of 183 cases of polio in Somalia, 14 in Kenya and 8 cases in the Somali Region of Ethiopia, but Africa had no confirmed cases of wild poliovirus (WPV) since 2016. Cases of circulating vaccine-derived poliovirus type 2 continue to appear in several countries.
On 25 August 2020, the Africa Regional Certification Commission declared Africa free from wild polio.
History
The effects of polio have been known since prehistory; Egyptian paintings and carvings depict otherwise healthy people with withered limbs, and children walking with canes at a young age. The first clinical description was provided by the English physician Michael Underwood in 1789, where he refers to polio as "a debility of the lower extremities". The work of physicians Jakob Heine in 1840 and Karl Oskar Medin in 1890 led to it being known as Heine–Medin disease. The disease was later called infantile paralysis, based on its propensity to affect children.
Before the 20th century, polio infections were rarely seen in infants before six months of age, most cases occurring in children six months to four years of age. Poorer sanitation of the time resulted in a constant exposure to the virus, which enhanced a natural immunity within the population. In developed countries during the late 19th and early 20th centuries, improvements were made in community sanitation, including better sewage disposal and clean water supplies. These changes drastically increased the proportion of children and adults at risk of paralytic polio infection, by reducing childhood exposure and immunity to the disease.
Small localized paralytic polio epidemics began to appear in Europe and the United States around 1900. Outbreaks reached pandemic proportions in Europe, North America, Australia, and New Zealand during the first half of the 20th century. By 1950, the peak age incidence of paralytic poliomyelitis in the United States had shifted from infants to children aged five to nine years, when the risk of paralysis is greater; about one-third of the cases were reported in persons over 15 years of age. Accordingly, the rate of paralysis and death due to polio infection also increased during this time. In the United States, the 1952 polio epidemic became the worst outbreak in the nation's history. Of the nearly 58,000 cases reported that year, 3,145 died and 21,269 were left with mild to disabling paralysis. Intensive care medicine has its origin in the fight against polio. Most hospitals in the 1950s had limited access to iron lungs for patients unable to breathe without mechanical assistance. Respiratory centers designed to assist the most severe polio patients, first established in 1952 at the Blegdam Hospital of Copenhagen by Danish anesthesiologist Bjørn Ibsen, were the precursors of modern intensive care units (ICU). (A year later, Ibsen would establish the world's first dedicated ICU.)
The polio epidemics not only altered the lives of those who survived them, but also brought profound cultural changes, spurring grassroots fund-raising campaigns that would revolutionize medical philanthropy, and giving rise to the modern field of rehabilitation therapy. As one of the largest disabled groups in the world, polio survivors also helped to advance the modern disability rights movement through campaigns for the social and civil rights of the disabled. The World Health Organization estimates that there are 10 to 20 million polio survivors worldwide. In 1977 there were 254,000 persons living in the United States who had been paralyzed by polio. According to doctors and local polio support groups, some 40,000 polio survivors with varying degrees of paralysis were living in Germany, 30,000 in Japan, 24,000 in France, 16,000 in Australia, 12,000 in Canada and 12,000 in the United Kingdom in 2001. Many notable individuals have survived polio and often credit the prolonged immobility and residual paralysis associated with polio as a driving force in their lives and careers.
The disease was very well publicized during the polio epidemics of the 1950s, with extensive media coverage of any scientific advancements that might lead to a cure. Thus, the scientists working on polio became some of the most famous of the century. Fifteen scientists and two laymen who made important contributions to the knowledge and treatment of poliomyelitis are honored by the Polio Hall of Fame, which was dedicated in 1957 at the Roosevelt Warm Springs Institute for Rehabilitation in Warm Springs, Georgia, US. In 2008 four organizations (Rotary International, the World Health Organization, the U.S. Centers for Disease Control and UNICEF) were added to the Hall of Fame.
World Polio Day (24 October) was established by Rotary International to commemorate the birth of Jonas Salk, who led the first team to develop a vaccine against poliomyelitis. Use of this inactivated poliovirus vaccine and subsequent widespread use of the oral poliovirus vaccine developed by Albert Sabin led to establishment of the Global Polio Eradication Initiative (GPEI) in 1988. Since then, GPEI has reduced polio worldwide by 99 percent.
Etymology
The term derives from the Ancient Greek poliós (πολιός), meaning "grey", myelós (µυελός "marrow"), referring to the grey matter of the spinal cord, and the suffix -itis, which denotes inflammation, i.e., inflammation of the spinal cord's grey matter, although a severe infection can extend into the brainstem and even higher structures, resulting in polioencephalitis, resulting in inability to breathe, requiring mechanical assistance such as an iron lung.