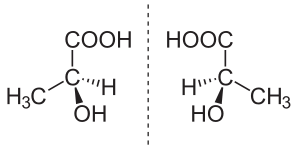
(S)-(+)-lactic acid (left) and (R)-(–)-lactic acid (right) are nonsuperposable mirror images of each other
A sample of a chemical is considered enantiopure (also termed enantiomerically pure) when it has, within the limits of detection, molecules of only one chirality.
When present in a symmetric environment, enantiomers have identical chemical and physical properties except for their ability to rotate plane-polarized light (+/−) by equal amounts but in opposite directions (although the polarized light can be considered an asymmetric medium). Such compounds are therefore described as optically active, with specific terms for each enantiomer based on the direction: a dextrorotatory compound rotates light a clockwise (+) direction whereas a levorotatory compound rotates light in a counter-clockwise (–) direction. A mixture of equal number of both enantiomers is called a racemic mixture or a racemate. In a racemic mixture, the amount of positive rotation is exactly counteracted by the equal amount of negative rotation, so the net rotation is zero (the mixture is not optically active). For all intents and purposes, pairs of enantiomers have the same Gibbs free energy. However, theoretical physics predicts that due to parity violation of the weak nuclear force (the only force in nature that can "tell left from right"), there is actually a minute difference in energy between enantiomers (on the order of 10−12 eV or 10−10 kJ/mol or less) due to the weak neutral current mechanism. This difference in energy is far smaller than energy changes caused by even a trivial change in molecular conformation and far too small to measure by current technology, and is therefore chemically inconsequential.
Enantiomer members often have different chemical reactions with other enantiomer substances. Since many biological molecules are enantiomers, there is sometimes a marked difference in the effects of two enantiomers on biological organisms. In drugs, for example, often only one of a drug's enantiomers is responsible for the desired physiological effects, while the other enantiomer is less active, inactive, or sometimes even productive of adverse effects. Owing to this discovery, drugs composed of only one enantiomer ("enantiopure") can be developed to make the drug work better and sometimes eliminate some side effects. An example is eszopiclone (Lunesta), which is just a single enantiomer of an older racemic drug called zopiclone. One enantiomer is responsible for all the desired effects, while the other enantiomer seems to be inactive, and the so the dose of eszopiclone is half that of zopiclone.
In chemical synthesis of enantiomeric substances, non-enantiomeric precursors inevitably produce racemic mixtures. In the absence of an effective enantiomeric environment (precursor, chiral catalyst, or kinetic resolution), separation of a racemic mixture into its enantiomeric components is impossible, although certain racemic mixtures spontaneously crystallize in the form of a racemic conglomerate, in which crystals of the enantiomers are physically segregated and may be separated mechanically (e.g., the enantiomers of tartaric acid, whose crystallized enantiomers were separated with tweezers by Pasteur). However, most racemates will crystallize in crystals containing both enantiomers in a 1:1 ratio, arranged in a regular lattice.
Naming conventions
The R/S system is an important nomenclature system used to denote
distinct enantiomers. Another system is based on prefix notation for
optical activity: (+)- and (−)- or d- and l-. The Latin words for left are laevus and sinister, and the word for right is dexter (or rectus in the sense of correct or virtuous). The English word right is a cognate of rectus. This is the origin of the L/D and S/R notations, and the employment of prefixes levo- and dextro- in common names.
Criterion of enantiomerism
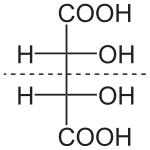
Fischer projection of meso-tartaric acid
An asymmetric carbon atom is one which has bonds with four different
atoms or groups, so that these bonds can be arranged in two different
ways which are not superposable. Most compounds that contain one or more
asymmetric carbon (or other element with a tetrahedral geometry) atoms
show enantiomerism, but this is not always true. Compounds that contain
two or more asymmetric carbon atoms but have a plane of symmetry with
respect to the whole molecule are known as meso compounds. A meso
compound does not have a mirror image stereoisomer because it is its
own mirror image (i.e., it and its mirror image are the same molecule).
For instance, meso tartaric acid
(shown on the right) has two asymmetric carbon atoms, but it does not
exhibit enantiomerism because each of the two halves of the molecule is
equal and opposite to the other and thus is superposable on its
geometric mirror image. Conversely, there exist forms of chirality that
do not require individual asymmetric atoms. In fact, there are four
distinct types of chirality: central, axial, planar, and helical
chirality. Having an enantiomer by virtue of an asymmetric carbon atom
represents the most common type of central chirality. The other three
types of chirality do not involve asymmetric carbon atoms, and even
central chirality does not require the center of chirality to be located
at a carbon or any other atom. Consequently, while the presence of an
asymmetric carbon atom is a convenient characteristic to look for when
determining whether a molecule will have an enantiomer, it is neither
sufficient nor necessary as a criterion.
As a rigorous criterion, a molecule is chiral, and will therefore
possess an enantiomer, if and only if it belongs to one of the chiral
point groups: Cn, Dn, T, O, and I.
However, as a caveat, enantiomers are not necessarily isolable if
there is an accessible pathway for racemization at a given temperature
and timescale. For example, amines with three distinct substituents are
chiral, but with the exception of only a few atypical cases (e.g.
substituted N-chloroaziridines), they rapidly planarize and
invert ("umbrella inversion") at room temperature, leading to
racemization. If the racemization is fast enough, the molecule can often
be treated as an achiral, averaged structure.
Examples
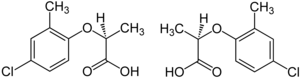
Structures of the two enantiomeric forms (S left, R right) of mecoprop
Enantiomers of citalopram. The top is (R)-citalopram and the bottom is (S)-citalopram.
An example of such an enantiomer is the sedative thalidomide,
which was sold in a number of countries around the world from 1957
until 1961. It was withdrawn from the market when it was found to cause
birth defects. One enantiomer caused the desirable sedative effects,
while the other, unavoidably present in equal quantities, caused birth defects.
The herbicide mecoprop is a racemic mixture, with the (R)-(+)-enantiomer ("Mecoprop-P", "Duplosan KV") possessing the herbicidal activity.
Another example is the antidepressant drugs escitalopram and citalopram. Citalopram is a racemate [1:1 mixture of (S)-citalopram and (R)-citalopram]; escitalopram [(S)-citalopram] is a pure enantiomer. The dosages for escitalopram are typically 1/2 of those for citalopram.
Enantioselective preparations
There are two main strategies for the preparation of enantiopure
compounds. The first is known as chiral resolution. This method involves
preparing the compound in racemic form, and separating it into its isomers. In his pioneering work, Louis Pasteur was able to isolate the isomers of tartaric acid because they crystallize from solution as crystals each with a different symmetry. A less common method is by enantiomer self-disproportionation.
The second strategy is asymmetric synthesis: the use of various techniques to prepare the desired compound in high enantiomeric excess. Techniques encompassed include the use of chiral starting materials (chiral pool synthesis), the use of chiral auxiliaries and chiral catalysts, and the application of asymmetric induction. The use of enzymes (biocatalysis) may also produce the desired compound.
Enantioconvergent synthesis
is the synthesis of one enantiomer from a racemic precursor molecule
utilizing both enantiomers. Thus, the two enantiomers of the reactant
produce a single enantiomer of product.
Enantiopure medications
Advances in industrial chemical processes have made it economic for
pharmaceutical manufacturers to take drugs that were originally marketed
as a racemic mixture
and market the individual enantiomers. In some cases, the enantiomers
have genuinely different effects. In other cases, there may be no
clinical benefit to the patient. In some jurisdictions,
single-enantiomer drugs are separately patentable from the racemic
mixture.
It is possible that only one of the enantiomers is active. Or, it may
be that both are active, in which case separating the mixture has no
objective benefits, but extends the drug's patentability.
Quasi-enantiomers
Quasi-enantiomers are molecular species that are not strictly enantiomers, but behave as if they are. Quasi-enantiomers have applications in parallel kinetic resolution.