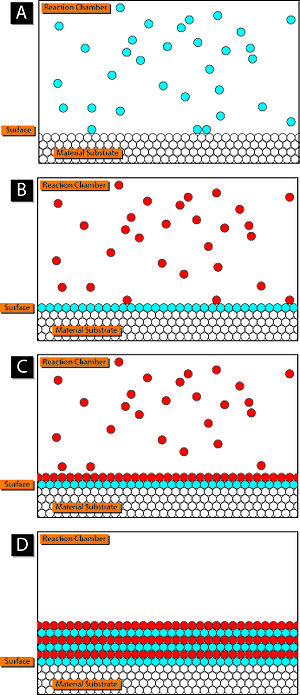
A
basic schematic of the atomic layer deposition process. In Frame A,
precursor 1 (in blue) is added to the reaction chamber containing the
material surface to be coated ALD. After precursor 1 has adsorbed on the
surface, any excess is removed from the reaction chamber. Precursor 2
(red) is added (Frame B) and reacts with precursor 1 to create another
layer on the surface (Frame C). Precursor 2 is then cleared from the
reaction chamber and this process is repeated until a desired thickness
is achieved and the resulting product resembles Frame D.
Atomic layer deposition (ALD) is a thin-film deposition technique based on the sequential use of a gas phase chemical process. ALD is considered a subclass of chemical vapor deposition. The majority of ALD reactions use two chemicals, typically called precursors.
These precursors react with the surface of a material one at a time in a
sequential, self-limiting, manner. Through the repeated exposure to
separate precursors, a thin film is slowly deposited. ALD is a key
process in the fabrication of semiconductor devices, and part of the set of tools
available for the synthesis of nanomaterials.
Introduction
Atomic
layer deposition (ALD) is a thin-film deposition method in which a film
is grown on a substrate by exposing its surface to alternate gaseous
species (typically referred to as precursors). In contrast to chemical vapor deposition,
the precursors are never present simultaneously in the reactor, but
they are inserted as a series of sequential, non-overlapping pulses. In
each of these pulses the precursor molecules react with the surface in a
self-limiting way, so that the reaction terminates once all the
reactive sites on the surface are consumed. Consequently, the maximum
amount of material deposited on the surface after a single exposure to
all of the precursors (a so-called ALD cycle) is determined by the
nature of the precursor-surface interaction.
By varying the number of cycles it is possible to grow materials
uniformly and with high precision on arbitrarily complex and large
substrates.
ALD is considered one deposition method with great potential for
producing very thin, conformal films with control of the thickness and
composition of the films possible at the atomic level. A major driving
force for the recent interest is the prospective seen for ALD in scaling
down microelectronic devices according to Moore's law. ALD is an active field of research, with hundreds of different processes published in the scientific literature, though some of them exhibit behaviors that depart from that of an ideal ALD process.
History
ALD has been developed in two independent discoveries under names atomic layer epitaxy (ALE, Finland) and molecular layering (ML, Soviet Union).
To clarify the early history, an open effort called the Virtual Project
on the History of ALD (VPHA) has been set up in summer 2013 by a group
of scientists. Results from VPHA are dedicated essays that describe the historical development of ALD under the names ALE and ML; a review article that presents a short recommended reading list of early ALD publications up to 1986; and an article of learnings from the VPHA.
In the 1960s, Stanislav Ivanovich Koltsov together with Valentin Borisovich Aleskovskii and colleagues experimentally developed the principles of ALD at Leningrad (Lensovet) Technological Institute (LTI) in the Soviet Union.
The purpose was to experimentally build upon the theoretical
considerations of the "framework hypothesis" coined by Valentin
Borisovich Aleskovskii in his doctor of science thesis ("professor's
thesis") published in 1952.
The experiments started with metal chloride reactions and water with
porous silica, soon extending to other substrate materials and planar
thin films. Aleskovskii and Koltsov together proposed the name "Molecular Layering" for the new technique in 1965. The principles of Molecular Layering were summarized in the doctoral thesis ("professor's thesis") of Koltsov in 1971.
Research activities of molecular layering covered a broad scope, from
fundamental chemistry research to applied research with porous
catalysts, sorbents and fillers to microelectronics and beyond.
In 1974, when starting the development of thin-film electroluminescent displays (TFEL) at Instrumentarium Oy in Finland, Tuomo Suntola devised ALD as an advanced thin-film technology. Suntola named it atomic layer epitaxy (ALE) based on the meaning of "epitaxy" in Greek language, "arrangement upon". The first experiments were made with elemental Zn and S to grow ZnS. ALE as a means for growth of thin films was internationally patented in more than 20 countries.
A breakthrough occurred, when Suntola and co-workers switched from high
vacuum reactors to inert gas reactors which enabled the use of compound
reactants like metal chlorides, hydrogen sulphide and water vapor for
performing the ALE process. The technology was first disclosed in 1980 SID conference.
The TFEL display prototype presented consisted of a ZnS layer between
two aluminum oxide dielectric layers, all made in an ALE process using
ZnCl2 + H2S and AlCl3 + H2O
as the reactants. The first large-scale proof-of-concept of ALE-EL
displays were the flight information boards installed in the Helsinki-Vantaa airport in 1983. TFEL flat panel display production started in the mid-1980s by Lohja Oy in the Olarinluoma factory. Academic research on ALE started in Tampere University of Technology (where Suntola gave lectures on electron physics) in 1970s, and in 1980s at Helsinki University of Technology.
TFEL display manufacturing remained until the 1990s the only industrial
application of ALE. In 1987, Suntola started the development of the ALE
technology for new applications like photovoltaic devices and heterogeneous catalysts in Microchemistry Ltd., established for that purpose by the Finnish national oil company Neste
Oy. In the 1990s, ALE development in Microchemistry was directed to
semiconductor applications and ALE reactors suitable for silicon wafer
processing. In 1999, Microchemistry Ltd. and the ALD technology were
sold to the Dutch ASM International,
a major supplier of semiconductor manufacturing equipment and
Microchemistry Ltd. became ASM Microchemistry Oy as ASM's Finnish
daughter company. Microchemistry Ltd/ASM Microchemistry Ltd was the only
manufacturer of commercial ALD-reactors in the 1990s. In the early
2000s, the expertise on ALD reactors in Finland triggered two new
manufacturers, Beneq Oy and Picosun Oy, the latter started by Sven
Lindfors, Suntola's close coworker since 1975. The number of reactor
manufacturers increased rapidly and semiconductor applications became
the industrial breakthrough of the ALD technology, as ALD became an
enabling technology for the continuation of Moore's law. In 2004, Tuomo Suntola received the European SEMI award for the development of the ALD technology for semiconductor applications and in 2018 the Millennium Technology Prize.
The developers of ML and ALE met at the 1st international conference on atomic layer epitaxy, "ALE-1" in Espoo, Finland, 1990.
For some reason, knowledge of molecular layering in the growing
English-speaking ALD community has remained marginal. An attempt to
expose the extent of molecular layering works was made in a scientific
ALD review article in 2005 and later in the VPHA-related publications.
The name "atomic layer deposition" was apparently proposed for
the first time in writing as an alternative to ALE in analogy with CVD by Markku Leskelä (professor at the University of Helsinki)
at the ALE-1 conference, Espoo, Finland. It took about a decade, before
the name gained general acceptance with the onset of the international
conference series on Atomic Layer Deposition by American Vacuum Society.
Surface reaction mechanisms
In
a prototypical ALD process, a substrate is exposed to two reactants A
and B in a sequential, non-overlapping way. In contrast to other
techniques such as chemical vapor deposition
(CVD), where thin-film growth proceeds on a steady-state fashion, in
ALD each reactant reacts with the surface in a self-limited way: the
reactant molecules can react only with a finite number of reactive sites
on the surface. Once all those sites have been consumed in the reactor,
the growth stops. The remaining reactant molecules are flushed away and
only then reactant B is inserted into the reactor. By alternating
exposures of A and B, a thin film is deposited. This process is shown in
the side figure. Consequently, when describing an ALD process one
refers to both dose times (the time a surface is being exposed to a
precursor) and purge times (the time left in between doses for the
precursor to evacuate the chamber) for each precursor. The
dose-purge-dose-purge sequence of a binary ALD process constitutes an
ALD cycle. Also, rather than using the concept of growth rate, ALD
processes are described in terms of their growth per cycle.
In ALD, enough time must be allowed in each reaction step so that
a full adsorption density can be achieved. When this happens the
process has reached saturation. This time will depend on two key
factors: the precursor pressure, and the sticking probability. Therefore, the rate of adsorption per unit of surface area can be expressed as:
Where R is the rate of adsorption, S is the sticking probability, and F is the incident molar flux.[15]
However, a key characteristic of ALD is the S will change with time, as
more molecules have reacted with the surface this sticking probability
will become smaller until reaching a value of zero once saturation is
reached.
The specific details on the reaction mechanisms
are strongly dependent on the particular ALD process. With hundreds of
process available to deposit oxide, metals, nitrides, sulfides,
chalcogenides, and fluoride materials, the unraveling of the mechanistic aspects of ALD processes is an active field of research. Some representative examples are shown below.
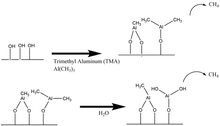
Thermal Al2O3 ALD
Among the different processes published in the literature, the synthesis of Al2O3 from trimethylaluminum (TMA) and water is one of the better known, and the self-limited growth of Al2O3 can be achieved in a wide range of temperature ranging from room temperature to more than 300 °C.
During the TMA exposure, TMA dissociatively chemisorbs on the
substrate surface and any remaining TMA is pumped out of the chamber.
The dissociative chemisorption of TMA leaves a surface covered with AlCH3.
The surface is then exposed to H2O vapor, which reacts with the surface –CH3 forming CH4 as a reaction byproduct and resulting on a hydroxylated Al2O3 surface.
Proposed Mechanism for Al2O3 ALD during the a) TMA reaction b) H2O reaction
Metal ALD
Metal ALD via elimination reactions most commonly occurs when metals functionalized with halogens
(i.e. metal fluorides) are reacted with silicon precursors. Common
metals deposited using fluorosilane elimination reactions are tungsten
and molybdenum because the respective elimination reactions for these
metals are highly exothermic
For Tungsten ALD, Si–H and W–F entities exist on the material's surface
prior to the final purging process, and a linear deposition rate of W
has been observed per each AB reactant cycle. Typical growth rates per
cycle for Tungsten ALD are 4 to 7 Angstroms and typical reaction
temperatures are 177 °C to 325 °C. Two surface reactions, as well as an
overall ALD reaction for tungsten ALD, are presented below. A multitude
of other metals can be deposited by ALD via the reactions below if their
reaction sequences are based on fluorosilane elimination.
- Primary Reactions at Surface:
- WSiF2H* + WF6--> WWF5* + SiF3H (7)
- WF5* + Si2H6 --> WSiF2H* + SiF3H + 2H2 (8)
- Overall ALD Reaction:
- WF6 + Si2H6 --> W + 2SiF3H + 2H2 ∆H = -181kcal (9)
Catalytic SiO2 ALD
The use of catalysts is of paramount importance in delivering reliable methods of SiO2 ALD. Without catalysts, surface reactions leading to the formation of SiO2 are generally very slow and only occur at exceptionally high temperatures. Typical catalysts for SiO2 ALD include Lewis bases such as NH3 or pyridine and SiO2 ; ALD can also be initiated when these Lewis bases are coupled with other silicon precursors such as tetraethoxysilane (TEOS). Hydrogen bonding is believed to occur between the Lewis base and the SiOH* surface species or between the H2O based reactant and the Lewis base. Oxygen becomes a stronger nucleophile
when the Lewis base hydrogen bonds with the SiOH* surface species
because the SiO-H bond is effectively weakened. As such, the
electropositive Si atom in the SiCl4 reactant is more susceptible to nucleophilic attack. Similarly, hydrogen bonding between a Lewis base and an H2O reactant make the electronegative O in H2O a strong nucleophile that is able to attack the Si in an existing SiCl* surface species. The use of a Lewis base catalyst is more or less a requirement for SiO2 ALD, as without a Lewis base catalyst, reaction temperatures must exceed 325 °C and pressures must exceed 103 torr. Generally, the most favorable temperature to perform SiO2 ALD is at 32 °C and a common deposition rate is 1.35 Angstroms per binary reaction sequence. Two surface reactions for SiO2 ALD, an overall reaction, and a schematic illustrating Lewis base catalysis in SiO2 ALD are provided below.
- Primary Reactions at Surface:
- SiOH* + SiCl4--> SiOSiCl3* + HCl (4)
- SiCl* + H2O --> SiOH* + HCl (5)
- Overall ALD Reaction:
- SiCl4 + 2H2O --> SiO2 + 4HCl (6)
- SiCl4 + 2H2O --> SiO2 + 4HCl (6)
Proposed Mechanism of Lewis base catalysis of SiO2 ALD during a) an SiCl4 reaction and b) an H2O reaction
| Type of ALD | Temperature range | Viable precursors | Reactants | Applications |
|---|---|---|---|---|
| Catalytic ALD | >32 °C with Lewis Base Catalyst | Metal oxides (i.e. TiO2, ZrO2,SnO22) | (Metal)Cl4, H2O | High k-dielectric layers, protective layers, anti-reflective layers, etc. |
| Al2O3 ALD | 30–300 °C | Al2O3, metal oxides | (Metal)Cl4, H2O, Ti(OiPr)4, (Metal)(Et)2 |
Dielectric layers, insulating layers, etc., Solar Cell surface passivations |
| Metal ALD Using Thermal Chemistry | 175–400 °C | Metal Fluorides, organometallics, catalytic metals | M(C5H5)2, (CH3C5H4)M(CH3)3 , Cu(thd)2, Pd(hfac)2, Ni(acac)2, H2 |
Conductive pathways, catalytic surfaces, MOS devices |
| ALD on polymers | 25–100 °C | Common polymers (Polyethylene, PMMA, PP, PS, PVC, PVA, etc.) | Al(CH3)3, H2O, M(CH3)3 | Polymer surface functionalization, creation of composites, diffusion barriers, etc. |
| ALD on particles | Varies: 25–100 deg C for polymer particles, 100–400 deg C for metal/alloy particles | BN, ZrO2, CNTs, polymer particles | Various gases: Fluidized bed reactors are used to allow coating of individual particles |
Deposition of protective and insulative coatings, optical and mechanical property modification, formation of composite structures, conductive mediums |
| Plasma or Radical-enhanced ALD for single element ALD materials | 20–800 °C | Pure metals (i.e. Ta, Ti, Si, Ge, Ru, Pt), metal nitrides (i.e. TiN, TaN, etc.) | Organometallics, MH2Cl2, tertbutylimidotris(diethylamido) tantalum (TBTDET), bis(ethylcyclopentadienyl) ruthenium), NH3 |
DRAM structures, MOSFET and semiconductor devices, capacitors |
| Plasma Enhanced ALD of Metal Oxides and Nitrides | 20–300 °C | Al2O3, SiO2, ZnOx, InOx, HfO2, SiNx, TaNx | similar to thermal ALD |
|
Applications
ALD
can be used for a great deal of applications. Some of the main fields
that ALD is used for are microelectronics and biomedical applications.
Details about these applications are outlined in the following sections.
Microelectronics applications
ALD
is a useful process for the fabrication of microelectronics due to its
ability to produce accurate thicknesses and uniform surfaces in addition
to high quality film production using various different materials. In
microelectronics, ALD is studied as a potential technique to deposit high-κ (high permittivity)
gate oxides, high-κ memory capacitor dielectrics, ferroelectrics, and
metals and nitrides for electrodes and interconnects. In high-κ gate
oxides, where the control of ultra thin films is essential, ALD is only
likely to come into wider use at the 45 nm technology. In
metallizations, conformal films are required; currently it is expected
that ALD will be used in mainstream production at the 65 nm node. In dynamic random access memories
(DRAMs), the conformality requirements are even higher and ALD is the
only method that can be used when feature sizes become smaller than
100 nm. Several products that use ALD include magnetic recording heads, MOSFET gate stacks, DRAM capacitors, nonvolatile ferroelectric memories, and many others.
Gate oxides
Deposition of the high-κ oxides Al2O3, ZrO2, and HfO2
has been one of the most widely examined areas of ALD. The motivation
for high-κ oxides comes from the problem of high tunneling current
through the commonly used SiO2 gate dielectric in metal-oxide-semiconductor field-effect transistors
(MOSFETs) when it is downscaled to a thickness of 1.0 nm and below.
With the high-κ oxide, a thicker gate dielectric can be made for the
required capacitance density, thus the tunneling current can be reduced
through the structure.
Intel Corporation has reported using ALD to deposit high-κ gate dielectric for its 45 nm CMOS technology.
Transition-metal nitrides
Transition-metal nitrides, such as TiN and TaN find potential use both as metal barriers and as gate metals. Metal barriers are used in modern Cu-based
chips to avoid diffusion of Cu into the surrounding materials, such as
insulators and the silicon substrate, and also, to prevent Cu
contamination by elements diffusing from the insulators by surrounding
every Cu interconnection with a layer of metal barriers. The metal
barriers have strict demands: they should be pure; dense; conductive;
conformal; thin; have good adhesion towards metals and insulators. The
requirements concerning process technique can be fulfilled by ALD. The
most studied ALD nitride is TiN which is deposited from TiCl4 and NH3.
Metal films
Motivations of an interest in metal ALD are:
- Cu interconnects and W plugs, or at least Cu seed layers for Cu electrodeposition and W seeds for W CVD,
- transition-metal nitrides (e.g. TiN, TaN, WN) for Cu interconnect barriers
- noble metals for ferroelectric random access memory (FRAM) and DRAM capacitor electrodes
- high- and low-work function metals for dual-gate MOSFETs.
Magnetic recording heads
Magnetic recording heads utilize electric fields to polarize particles and leave a magnetized pattern on a hard disk. Al2O3 ALD is used to create uniform, thin layers of insulation.
By using ALD, it is possible to control the insulation thickness to a
high level of accuracy. This allows for more accurate patterns of
magnetized particles and thus higher quality recordings.
DRAM capacitors
Dynamic
random-access memory (DRAM) capacitors are yet another application of
ALD. An individual DRAM cell can store a single bit of data and
consists of a single MOS transistor and a capacitor.
Major efforts are being put into reducing the size of the capacitor
which will effectively allow for greater memory density. In order to
change the capacitor size without affecting the capacitance, different
cell orientations are being used. Some of these include stacked or
trench capacitors.
With the emergence of trench capacitors, the problem of fabricating
these capacitors comes into play, especially as the size of semiconductors
decreases. ALD allows trench features to be scaled to beyond 100 nm.
The ability to deposit single layers of material allows for a great deal
of control over the material. Except for some issues of incomplete film
growth (largely due to insufficient amount or low temperature
substrates), ALD provides an effective means of depositing thin films
like dielectrics or barriers.
Biomedical applications
Understanding and being able to specify the surface properties on biomedical
devices is critical in the biomedical industry, especially regarding
devices that are implanted in the body. A material interacts with the
environment at its surface, so the surface properties largely direct the
interactions of the material with its environment. Surface chemistry and surface topography affect protein adsorption, cellular interactions, and the immune response.
Some current uses in biomedical applications include creating
flexible sensors, modifying nanoporous membranes, polymer ALD, and
creating thin biocompatible coatings. ALD has been used to deposit TiO2 films to create optical waveguide sensors as diagnostic tools.
Also, ALD is beneficial in creating flexible sensing devices that can
be used, for example, in the clothing of athletes to detect movement or
heart rate. ALD is one possible manufacturing process for flexible
organic field-effect transistors (OFETs) because it is a low-temperature
deposition method.
Nanoporous
materials are emerging throughout the biomedical industry in drug
delivery, implants, and tissue engineering. The benefit of using ALD to
modify the surfaces of nanoporous materials is that, unlike many other
methods, the saturation and self-limiting nature of the reactions means
that even deeply embedded surfaces and interfaces are coated with a
uniform film. Nanoporous surfaces can have their pore size reduced
further in the ALD process because the conformal coating will completely
coat the insides of the pores. This reduction in pore size may be
advantageous in certain applications.
Quality and quality control
The
quality of an ALD process can be monitored using several different
imaging techniques to make sure that the ALD process is occurring
smoothly and producing a conformal layer over a surface. One option is
cross-sectional SEM images or transmission electron microscopy (TEM)
images, which allow for inspection at the micro and nano scale. High
magnification of images is pertinent for assessing the quality of an ALD
layer. XRR, or X-ray reflectivity, is a technique that measures
thin-film properties including thickness, density, and surface
roughness.
Another optical quality evaluation tool is spectroscopic ellipsometry
(SE). Using SE in between the depositions of each layer added on by ALD
provides information on the growth rate and material characteristics of
the film can be assessed.
Applying this analysis tool during the ALD process, sometimes
referred to as in situ spectroscopic ellipsometry, allows for greater
control over the growth rate of the films during the ALD process. This
type of quality control occurs during the ALD process rather than
assessing the films afterwards as in TEM imaging, or XRR. Additionally,
Rutherford backscattering spectroscopy (RBS), X-Ray photoelectron
spectroscopy (XPS), Auger electron spectroscopy (AES), and four-point
probe (FPP) are some other techniques that can be used to provide
quality control information with regards to thin films deposited by ALD.
Advantages and limitations
Advantages
ALD
provides a very controlled method to produce a film to an atomically
specified thickness. Also, the growth of different multilayer structures
is straightforward. Due to the sensitivity and precision of the
equipment, it is very beneficial to those in the field of
microelectronics and nanotechnology in producing small, but efficient
semiconductors. ALD is typically run at lower temperatures along with a
catalyst which is thermochemically favored. The lower temperature is
beneficial when working with fragile substrates, such as biological
samples. Some precursors that are thermally unstable still may be used
so long as their decomposition rate is relatively slow.
Disadvantages
High
purity of the substrates is very important, and as such, high costs
will ensue (Stanford). Although this cost may not be much relative to
the cost of the equipment needed, one may need to run several trials
before finding conditions that favor their desired product. Once the
layer has been made and the process is complete, there may be a
requirement of needing to remove excess precursors from the final
product. In some final products there are less than one percent of
impurities present.
Economic viability
Atomic
layer deposition instruments can range anywhere from $200,000 to
$800,000 based on the quality and efficiency of the instrument. There is
no set cost for running a cycle of these instruments; the cost varies
depending on the quality and purity of the substrates used, as well as
the temperature and time of machine operation. Some substrates are less
available than others and require special conditions, as some are very
sensitive to oxygen and may then increase the rate of decomposition.
Multicomponent oxides and certain metals traditionally needed in the
microelectronics industry are generally not cost efficient.
Reaction time
The
process of ALD is very slow and this is known to be its major
limitation. For example, Al2O3 is deposited at a rate of 0.11 nm per
cycle,
which can correspond to an average deposition rate of 100–300 nm per
hour, depending on cycle duration and pumping speed. ALD is typically
used to produce substrates for microelectronics and nanotechnology, and
therefore, thick atomic layers are not needed. Many substrates cannot be
used because of their fragility or impurity. Impurities are typically
found on the 0.1-1% atomic level because of some of the carrier gases
are known to leave residue and are also sensitive to oxygen.
Chemical limitations
Precursors
must be volatile, but not subject to decomposition, as most precursors
are very sensitive to oxygen/air, thus causing a limitation on the
substrates that may be used. Some biological substrates are very
sensitive to heat and may have fast decomposition rates that are not
favored and yield larger impurity levels. There are a multitude of
thin-film substrate materials available, but the important substrates
needed for use in microelectronics can be hard to obtain and may be very
expensive.