Diagram showing a generalized view of cellular iron homeostasis in humans. Iron import can occur via endocytosis of transferrin receptor 1 or via ferrous iron importers DMT1 and ZIP14, which require the activity of iron reductases such as STEAP2, SDR-2 and Dcytb. Intracellular iron can be stored in ferritin, used for protein biosynthesis, generate reactive oxygen species (ROS) and regulate transcription via iron-responsive element-binding proteins (IRP1/2). Export occurs through ferroportin, often aided by hephaestin (Hp) and/or ceruloplasmin (Cp), and repressed by hepcidin.
Human iron metabolism is the set of chemical reactions that maintain human homeostasis of iron
at the systemic and cellular level. Iron is both necessary to the body
and potentially toxic, and controlling iron levels in the body is a
critically important part of many aspects of human health and disease. Hematologists have been especially interested in systemic iron metabolism because iron is essential for red blood cells,
where most of the human body's iron is contained. Understanding iron
metabolism is also important for understanding diseases of iron overload, such as hereditary hemochromatosis, and iron deficiency, such as iron deficiency anemia.
Importance of iron regulation
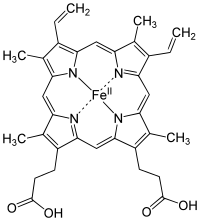
Structure of Heme b; "Fe" is the chemical symbol of iron, "II" indicates its oxidation state.
Iron is an essential bioelement for most forms of life, from bacteria to mammals. Its importance lies in its ability to mediate electron transfer. In the ferrous state, iron acts as an electron donor, while in the ferric state it acts as an acceptor. Thus, iron plays a vital role in the catalysis of enzymatic reactions that involve electron transfer (reduction and oxidation, redox). Proteins can contain iron as part of different cofactors, such as iron-sulfur clusters (Fe-S) and heme groups, both of which are assembled in mitochondria.
Cellular respiration
Human cells require iron in order to obtain energy as ATP from a multi-step process known as cellular respiration, more specifically from oxidative phosphorylation at the mitochondrial cristae. Iron is present in the iron-sulfur clusters and heme groups of the electron transport chain proteins that generate a proton gradient that allows ATP synthase to synthesize ATP (chemiosmosis).
Heme groups are part of hemoglobin,
a protein found in red blood cells that serves to transport oxygen from
the lungs to the tissues. Heme groups are also present in myoglobin to store and diffuse oxygen in muscle cells.
Oxygen transport
The human body needs iron for oxygen transport. Oxygen (O2)
is required for the functioning and survival of nearly all cell types.
Oxygen is transported from the lungs to the rest of the body bound to
the heme group of hemoglobin in erythrocytes. In muscles cells, iron binds myoglobin, which regulates its release.
Toxicity
Iron is also potentially toxic. Its ability to donate and accept electrons means that it can catalyze the conversion of hydrogen peroxide into free radicals. Free radicals can cause damage to a wide variety of cellular structures, and ultimately kill the cell.
Iron bound to proteins or cofactors such as heme
is safe. Also, there are virtually no truly free iron ions in the
cell, since they readily form complexes with organic molecules. However,
some of the intracellular iron is bound to low-affinity complexes, and
is termed labile iron or "free" iron. Iron in such complexes can cause
damage as described above.
To prevent that kind of damage, all life forms that use iron bind the iron atoms to proteins. This binding allows cells to benefit from iron while also limiting its ability to do harm. Typical intracellular labile iron concentrations in bacteria are 10-20 micromolar, though they can be 10-fold higher in anaerobic environment, where free radicals and reactive oxygen species
are scarcer. In mammalian cells, intracellular labile iron
concentrations are typically smaller than 1 micromolar, less than 5
percent of total cellular iron.
Bacterial protection
Electron micrograph of E. coli. Most bacteria that cause human disease require iron to live and to multiply.
In response to a systemic bacterial infection, the immune system initiates a process known as iron withholding.
If bacteria are to survive, then they must obtain iron from their
environment. Disease-causing bacteria do this in many ways, including
releasing iron-binding molecules called siderophores and then reabsorbing them to recover iron, or scavenging iron from hemoglobin and transferrin. The harder they have to work to get iron, the greater a metabolic price
they must pay. That means that iron-deprived bacteria reproduce more
slowly. So our control of iron levels appears to be an important defense
against most bacterial infections; there are some exceptions however. TB causing bacterium can reside within macrophages which are an iron rich environment and Borrelia burgdorferi utilises manganese
in place of iron. People with increased amounts of iron, like people
with hemochromatosis, are more susceptible to some bacterial infection.
Although this mechanism is an elegant response to short-term
bacterial infection, it can cause problems when inflammation goes on for
longer. Since the liver produces hepcidin in response to inflammatory cytokines,
hepcidin levels can increase as the result of non-bacterial sources of
inflammation, like viral infection, cancer, auto-immune diseases or
other chronic diseases. When this occurs, the sequestration of iron
appears to be the major cause of the syndrome of anemia of chronic disease, in which not enough iron is available to produce enough hemoglobin-containing red blood cells.
Body iron stores
Illustration of blood cell production in the bone marrow. In iron deficiency, the bone marrow produces fewer blood cells, and as the deficiency gets worse, the cells become smaller.
Most well-nourished people in industrialized countries have 4 to
5 grams of iron in their bodies (∼38 mg iron/kg body weight for women
and ∼50 mg iron/kg body for men). Of this, about 2.5 g
is contained in the hemoglobin needed to carry oxygen through the
blood, and most of the rest (approximately 2 grams in adult men, and
somewhat less in women of childbearing age) is contained in ferritin complexes that are present in all cells, but most common in bone marrow, liver, and spleen. The liver's stores of ferritin are the primary physiologic source of
reserve iron in the body. The reserves of iron in industrialized
countries tend to be lower in children and women of child-bearing age
than in men and in the elderly. Women who must use their stores to
compensate for iron lost through menstruation, pregnancy or lactation have lower non-hemoglobin body stores, which may consist of 500 mg, or even less.
Of the body's total iron content, about 400 mg
is devoted to cellular proteins that use iron for important cellular
processes like storing oxygen (myoglobin) or performing energy-producing
redox reactions (cytochromes). A relatively small amount (3–4 mg) circulates through the plasma, bound to transferrin. Because of its toxicity, free soluble iron is kept in low concentration in the body.
Iron deficiency first affects the storage iron in the body, and
depletion of these stores is thought to be relatively non-symptomatic,
although some vague and non-specific symptoms
have been associated with it. Since iron is primarily required for
hemoglobin, iron deficiency anemia is the primary clinical manifestation
of iron deficiency. Iron-deficient people will suffer or die from organ
damage well before cells run out of the iron needed for intracellular
processes like electron transport.
Macrophages of the reticuloendothelial system
store iron as part of the process of breaking down and processing
hemoglobin from engulfed red blood cells. Iron is also stored as a
pigment called hemosiderin
which is an ill-defined deposit of protein and iron, created by
macrophages where excess iron is present, either locally or systemically
for example among people with iron overload due to frequent blood cell
destruction and transfusions. If the systemic iron overload is
corrected, over time the hemosiderin is slowly resorbed by macrophages.
Mechanisms of iron regulation
Human
iron homeostasis is regulated at two different levels. Systemic iron
levels are balanced by the controlled absorption of dietary iron by enterocytes, the cells that line the interior of the intestines,
and the uncontrolled loss of iron from epithelial sloughing, sweat,
injuries and blood loss. In addition, systemic iron is continuously
recycled. Cellular iron levels are controlled differently by different
cell types due to the expression of particular iron regulatory and
transport proteins.
Systemic iron regulation
Humans use 20 mg of iron each day for the production of new red blood cells, much of which is recycled from old red blood cells.
Dietary iron uptake
The
absorption of dietary iron is a variable and dynamic process. The
amount of iron absorbed compared to the amount ingested is typically
low, but may range from 5% to as much as 35% depending on circumstances
and type of iron. The efficiency with which iron is absorbed varies
depending on the source. Generally the best-absorbed forms of iron come
from animal products. Absorption of dietary iron in iron salt form (as
in most supplements) varies somewhat according to the body’s need for
iron, and is usually between 10% and 20% of iron intake. Absorption of
iron from animal products, and some plant products, is in the form of
heme iron, and is more efficient, allowing absorption of from 15% to 35%
of intake. Heme iron in animals is from blood and heme-containing
proteins in meat and mitochondria, whereas in plants, heme iron is
present in mitochondria in all cells that use oxygen for respiration.
Like most mineral nutrients, the majority of the iron absorbed from digested food or supplements is absorbed in the duodenum by enterocytes
of the duodenal lining. These cells have special molecules that allow
them to move iron into the body. To be absorbed, dietary iron can be
absorbed as part of a protein such as heme protein or iron must be in
its ferrous Fe2+ form. A ferric reductase enzyme on the enterocytes’ brush border, duodenal cytochrome B (Dcytb), reduces ferric Fe3+ to Fe2+. A protein called divalent metal transporter 1 (DMT1), which can transport several divalent metals across the plasma membrane, then transports iron across the enterocyte’s cell membrane into the cell. It the iron is bound to Heme it is instead transported across the apical membrane by Heme carrier protein 1 (HCP1).
These intestinal lining cells can then either store the iron as ferritin, which is accomplished by Fe3+ binding to apoferritin (in which case the iron will leave the body when the cell dies and is sloughed off into feces), or the cell can release it into the body via the only known iron exporter in mammals, ferroportin. Hephaestin, a ferroxidase that can oxidize Fe2+ to Fe3+
and is found mainly in the small intestine, helps ferroportin transfer
iron across the basolateral end of the intestine cells. In contrast,
ferroportin is post-translationally repressed by hepcidin,
a 25-amino acid peptide hormone. The body regulates iron levels by
regulating each of these steps. For instance, enterocytes synthesize
more Dcytb, DMT1 and ferroportin in response to iron deficiency anemia. Iron absorption from diet is enhanced in the presence of vitamin C and diminished by excess calcium, zinc, or manganese.
The human body’s rate of iron absorption appears to respond to a
variety of interdependent factors, including total iron stores, the
extent to which the bone marrow is producing new red blood cells, the
concentration of hemoglobin in the blood, and the oxygen content of the
blood. The body also absorbs less iron during times of inflammation,
in order to deprive bacteria of iron. Recent discoveries demonstrate
that hepcidin regulation of ferroportin is responsible for the syndrome
of anemia of chronic disease.
Iron recycling and loss
Most
of the iron in the body is hoarded and recycled by the
reticuloendothelial system, which breaks down aged red blood cells. In
contrast to iron uptake and recycling, there is no physiologic
regulatory mechanism for excreting iron. People lose a small but steady amount by gastrointestinal blood loss, sweating and by shedding cells of the skin and the mucosal lining of the gastrointestinal tract. The total amount of loss for healthy people in the developed world amounts to an estimated average of 1 mg a day for men, and 1.5–2 mg a day for women with regular menstrual periods. People with gastrointestinal parasitic infections, more commonly found in developing countries, often lose more.
Those who cannot regulate absorption well enough get disorders of iron
overload. In these diseases, the toxicity of iron starts overwhelming
the body's ability to bind and store it.
Cellular iron regulation
Iron import
Most cell types take up iron primarily through receptor-mediated endocytosis via transferrin receptor 1 (TFR1), transferrin receptor 2 (TFR2) and GAPDH. TFR1 has a 30-fold higher affinity for transferrin-bound iron than TFR2 and thus is the main player in this process.
The higher order multifunctional glycolytic enzyme
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) also acts as a
transferrin receptor.
Transferrin-bound ferric iron is recognized by these transferrin
receptors, triggering a conformational change that causes endocytosis.
Iron then enters the cytoplasm from the endosome via importer DMT1 after
being reduced to its ferrous state by a STEAP family reductase.
Alternatively, iron can enter the cell directly via plasma
membrane divalent cation importers such as DMT1 and ZIP14 (Zrt-Irt-like
protein 14).
Again, iron enters the cytoplasm in the ferrous state after being
reduced in the extracellular space by a reductase such as STEAP2, STEAP3
(in erythrocytes), Dcytb (in enterocytes) and SDR2.
The labile iron pool
In the cytoplasm, ferrous iron is found in a soluble, chelatable state which constitutes the labile iron pool (~0.001 mM).
In this pool, iron is thought to be bound to low-mass compounds such as
peptides, carboxylates and phosphates, although some might be in a
free, hydrated form (aqua ions). Alternatively, iron ions might be bound to specialized proteins known as metallochaperones.
Specifically, poly-r(C)-binding proteins (PCBPs) appear to mediate
transfer of free iron to ferritin (for storage) and non-heme iron
enzymes (for use in catalysis).
The labile iron pool is potentially toxic due to iron's ability to
generate reactive oxygen species. Iron from this pool can be taken up by
mitochondria via mitoferrin to synthesize Fe-S clusters and heme groups.
The storage iron pool
Iron can be stored in ferritin as ferric iron due to the ferroxidase activity of the ferritin heavy chain. Dysfunctional ferritin may accumulate as hemosiderin, which can be problematic in cases of iron overload. The ferritin storage iron pool is much larger than the labile iron pool, ranging in concentration from 0.7 mM to 3.6 mM.
Iron export
Iron
export occurs in a variety of cell types, including neurons,
erythrocytes, macrophages and enterocytes. The latter two are especially
important since systemic iron levels depend upon them. There is only
one known iron exporter, ferroportin. It transports ferrous iron out of the cell, generally aided by ceruloplasmin and/or hephaestin (mostly in enterocytes), which oxidize iron to its ferric state so it can bind ferritin in the extracellular medium. Hepcidin
causes the internalization of ferroportin, decreasing iron export.
Besides, hepcidin seems to downregulate both TFR1 and DMT1 through an
unknown mechanism. Another player assisting ferroportin in effecting cellular iron export is GAPDH.
A specific post translationally modified isoform of GAPDH is recruited
to the surface of iron loaded cells where it recruits apo-transferrin in
close proximity to ferroportin so as to rapidly chelate the iron
extruded.
The expression of hepcidin, which only occurs in certain cell types such as hepatocytes,
is tightly controlled at the transcriptional level and it represents
the link between cellular and systemic iron homeostasis due to
hepcidin's role as "gatekeeper" of iron release from enterocytes into
the rest of the body. Erythroblasts produce erythroferrone, a hormone which inhibits hepcidin and so increases the availability of iron needed for hemoglobin synthesis.
Translational control of cellular iron
Although
some control exists at the transcriptional level, the regulation of
cellular iron levels is ultimately controlled at the translational level
by iron-responsive element-binding proteins IRP1 and especially IRP2. When iron levels are low, these proteins are able to bind to iron-responsive elements (IREs). IREs are stem loop structures in the untranslated regions (UTRs) of mRNA.
Both ferritin and ferroportin contain an IRE in their 5' UTRs, so
that under iron deficiency their translation is repressed by IRP2,
preventing the unnecessary synthesis of storage protein and the
detrimental export of iron. In contrast, TFR1 and some DMT1 variants
contain 3' UTR IREs, which bind IRP2 under iron deficiency, stabilizing
the mRNA, which guarantees the synthesis of iron importers.
Pathology
Iron deficiency
Iron is an important topic in prenatal care because women can sometimes become iron-deficient from the increased iron demands of pregnancy.
Functional or actual iron deficiency can result from a variety of causes. These causes can be grouped into several categories:
- Increased demand for iron, which the diet cannot accommodate.
- Increased loss of iron (usually through loss of blood).
- Nutritional deficiency. This can result due to a lack of dietary iron or consumption of foods that inhibit iron absorption. Absorption inhibition has been observed caused by phytates in bran, calcium from supplements or dairy products, and tannins from tea, although in all three of these studies the effect was small and the authors of the studies cited regarding bran and tea note that the effect will probably only have a noticeable impact when most iron is obtained from vegetable sources.
- Acid-reducing medications: Acid-reducing medications reduce the absorption of dietary iron. These medications are commonly used for gastritis, reflux disease, and ulcers. Proton pump inhibitors (PPIs), H2 antihistamines, and antacids will reduce iron metabolism.
- Damage to the intestinal lining. Examples of causes of this kind of damage include surgery involving the duodenum, or diseases like Crohn's or celiac sprue which severely reduce the surface area available for absorption.
- Inflammation leading to hepcidin-induced restriction on iron release from enterocytes (see above).
Iron overload
The body is able to substantially reduce the amount of iron it
absorbs across the mucosa. It does not seem to be able to entirely shut
down the iron transport process. Also, in situations where excess iron
damages the intestinal lining itself (for instance, when children eat a
large quantity of iron tablets produced for adult consumption), even
more iron can enter the bloodstream and cause a potentially deadly
syndrome of iron overload. Large amounts of free iron in the circulation
will cause damage to critical cells in the liver, the heart and other metabolically active organs.
Iron toxicity results when the amount of circulating iron exceeds
the amount of transferrin available to bind it, but the body is able to
vigorously regulate its iron uptake. Thus, iron toxicity from ingestion
is usually the result of extraordinary circumstances like iron tablet
over-consumption rather than variations in diet.
The type of acute toxicity from iron ingestion causes severe mucosal
damage in the gastrointestinal tract, among other problems.
Excess iron has been linked to some cancers. Of note, a recent study showed that breast cancer patients with low ferroportin
expression (leading to higher concentrations of intracellular iron)
survive for a shorter period of time on average. Conversely, high
ferroportin expression in breast cancer predicts 90% 10-year survival.
Chronic iron toxicity is usually the result of more chronic iron
overload syndromes associated with genetic diseases, repeated
transfusions or other causes. In such cases the iron stores of an adult
may reach 50 grams (10 times normal total body iron) or more. Classic
examples of genetic iron overload includes hereditary hemochromatosis (HH) and the more severe disease juvenile hemochromatosis (JH) caused by mutations in either the gene RGMc gene, a member of a three gene repulsive guidance molecule family, (also called hemojuvelin (HJV), and HFE2), Hemojuvelin,
or the HAMP gene that encodes (an iron regulatory peptide). The exact
mechanisms of most of the various forms of adult hemochromatosis, which
make up most of the genetic iron overload disorders, remain unsolved. So
while researchers have been able to identify genetic mutations causing
several adult variants of hemochromatosis, they now must turn their
attention to the normal function of these mutated genes.