Dichroic filters are created using optically transparent materials.
In the field of optics, transparency (also called pellucidity or diaphaneity) is the physical property
of allowing light to pass through the material without being scattered.
On a macroscopic scale (one where the dimensions investigated are much
larger than the wavelength of the photons in question), the photons can be said to follow Snell's Law. Translucency (also called translucence or translucidity)
is a superset of transparency: it allows light to pass through, but
does not necessarily (again, on the macroscopic scale) follow Snell's
law; the photons can be scattered at either of the two interfaces, or
internally, where there is a change in index of refraction.
In other words, a translucent medium allows the transport of light
while a transparent medium not only allows the transport of light but
allows for image formation. Transparent materials appear clear, with the
overall appearance of one color, or any combination leading up to a
brilliant spectrum of every color. The opposite property of translucency is opacity.
When light encounters a material, it can interact with it in several different ways. These interactions depend on the wavelength
of the light and the nature of the material. Photons interact with an
object by some combination of reflection, absorption and transmission.
Some materials, such as plate glass and clean water,
transmit much of the light that falls on them and reflect little of it;
such materials are called optically transparent. Many liquids and
aqueous solutions are highly transparent. Absence of structural defects
(voids, cracks, etc.) and molecular structure of most liquids are mostly
responsible for excellent optical transmission.
Materials which do not transmit light are called opaque. Many such substances have a chemical composition which includes what are referred to as absorption centers. Many substances are selective in their absorption of white light frequencies. They absorb certain portions of the visible spectrum
while reflecting others. The frequencies of the spectrum which are not
absorbed are either reflected or transmitted for our physical
observation. This is what gives rise to color. The attenuation of light of all frequencies and wavelengths is due to the combined mechanisms of absorption and scattering.
Transparency can provide almost perfect camouflage for animals able to achieve it. This is easier in dimly-lit or turbid seawater than in good illumination. Many marine animals such as jellyfish are highly transparent.
Comparisons of 1. opacity, 2. translucency, and 3. transparency; behind each panel is a star
Introduction
With regard to the absorption of light, primary material considerations include:
- At the electronic level, absorption in the ultraviolet and visible (UV-Vis) portions of the spectrum depends on whether the electron orbitals are spaced (or "quantized") such that they can absorb a quantum of light (or photon) of a specific frequency, and does not violate selection rules. For example, in most glasses, electrons have no available energy levels above them in range of that associated with visible light, or if they do, they violate selection rules, meaning there is no appreciable absorption in pure (undoped) glasses, making them ideal transparent materials for windows in buildings.
- At the atomic or molecular level, physical absorption in the infrared portion of the spectrum depends on the frequencies of atomic or molecular vibrations or chemical bonds, and on selection rules. Nitrogen and oxygen are not greenhouse gases because there is no absorption, but because there is no molecular dipole moment.
With regard to the scattering of light,
the most critical factor is the length scale of any or all of these
structural features relative to the wavelength of the light being
scattered. Primary material considerations include:
- Crystalline structure: whether or not the atoms or molecules exhibit the 'long-range order' evidenced in crystalline solids.
- Glassy structure: scattering centers include fluctuations in density or composition.
- Microstructure: scattering centers include internal surfaces such as grain boundaries, crystallographic defects and microscopic pores.
- Organic materials: scattering centers include fiber and cell structures and boundaries.
Light scattering in solids
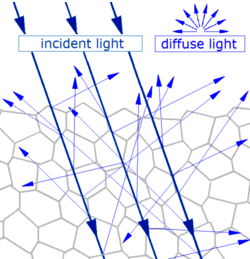
General mechanism of diffuse reflection
Diffuse reflection
- Generally, when light strikes the surface of a (non-metallic and
non-glassy) solid material, it bounces off in all directions due to
multiple reflections by the microscopic irregularities inside the material (e.g., the grain boundaries of a polycrystalline material, or the cell or fiber
boundaries of an organic material), and by its surface, if it is rough.
Diffuse reflection is typically characterized by omni-directional
reflection angles. Most of the objects visible to the naked eye are
identified via diffuse reflection. Another term commonly used for this
type of reflection is "light scattering". Light scattering from the
surfaces of objects is our primary mechanism of physical observation.
Light scattering in liquids and solids depends on the wavelength
of the light being scattered. Limits to spatial scales of visibility
(using white light) therefore arise, depending on the frequency of the
light wave and the physical dimension (or spatial scale) of the scattering center. Visible light has a wavelength scale on the order of a half a micrometer
(one millionth of a meter). Scattering centers (or particles) as small
as one micrometer have been observed directly in the light microscope (e.g., Brownian motion).
Transparent ceramics
Optical
transparency in polycrystalline materials is limited by the amount of
light which is scattered by their microstructural features. Light
scattering depends on the wavelength of the light. Limits to spatial
scales of visibility (using white light) therefore arise, depending on
the frequency of the light wave and the physical dimension of the
scattering center. For example, since visible light has a wavelength
scale on the order of a micrometer, scattering centers will have
dimensions on a similar spatial scale. Primary scattering centers in
polycrystalline materials include microstructural defects such as pores
and grain boundaries. In addition to pores, most of the interfaces in a
typical metal or ceramic object are in the form of grain boundaries
which separate tiny regions of crystalline order. When the size of the
scattering center (or grain boundary) is reduced below the size of the
wavelength of the light being scattered, the scattering no longer occurs
to any significant extent.
In the formation of polycrystalline materials (metals and
ceramics) the size of the crystalline grains is determined largely by
the size of the crystalline particles present in the raw material during
formation (or pressing) of the object. Moreover, the size of the grain
boundaries scales directly with particle size. Thus a reduction of the
original particle size well below the wavelength of visible light (about
1/15 of the light wavelength or roughly 600/15 = 40 nanometers) eliminates much of light scattering, resulting in a translucent or even transparent material.
Computer modeling of light transmission through translucent
ceramic alumina has shown that microscopic pores trapped near grain
boundaries act as primary scattering centers. The volume fraction of
porosity had to be reduced below 1% for high-quality optical
transmission (99.99 percent of theoretical density). This goal has been
readily accomplished and amply demonstrated in laboratories and research
facilities worldwide using the emerging chemical processing methods
encompassed by the methods of sol-gel chemistry and nanotechnology.
Translucency of a material being used to highlight the structure of a photographic subject
Transparent ceramics
have created interest in their applications for high energy lasers,
transparent armor windows, nose cones for heat seeking missiles,
radiation detectors for non-destructive testing, high energy physics,
space exploration, security and medical imaging applications. Large laser elements made from transparent ceramics can be produced at a relatively low cost. These components are free of internal stress or intrinsic birefringence,
and allow relatively large doping levels or optimized custom-designed
doping profiles. This makes ceramic laser elements particularly
important for high-energy lasers.
The development of transparent panel products will have other
potential advanced applications including high strength,
impact-resistant materials that can be used for domestic windows and
skylights. Perhaps more important is that walls and other applications
will have improved overall strength, especially for high-shear
conditions found in high seismic and wind exposures. If the expected
improvements in mechanical properties bear out, the traditional limits
seen on glazing areas in today's building codes could quickly become
outdated if the window area actually contributes to the shear resistance
of the wall.
Currently available infrared transparent materials typically
exhibit a trade-off between optical performance, mechanical strength and
price. For example, sapphire (crystalline alumina) is very strong, but it is expensive and lacks full transparency throughout the 3–5 micrometer mid-infrared range. Yttria
is fully transparent from 3–5 micrometers, but lacks sufficient
strength, hardness, and thermal shock resistance for high-performance
aerospace applications. Not surprisingly, a combination of these two
materials in the form of the yttrium aluminium garnet (YAG) is one of the top performers in the field.
Absorption of light in solids
When
light strikes an object, it usually has not just a single frequency (or
wavelength) but many. Objects have a tendency to selectively absorb,
reflect or transmit light of certain frequencies. That is, one object
might reflect green light while absorbing all other frequencies of
visible light. Another object might selectively transmit blue light
while absorbing all other frequencies of visible light. The manner in
which visible light interacts with an object is dependent upon the
frequency of the light, the nature of the atoms in the object, and often
the nature of the electrons in the atoms of the object.
Some materials allow much of the light that falls on them to be
transmitted through the material without being reflected. Materials that
allow the transmission of light waves through them are called optically
transparent. Chemically pure (undoped) window glass and clean river or
spring water are prime examples of this.
Materials which do not allow the transmission of any light wave frequencies are called opaque.
Such substances may have a chemical composition which includes what are
referred to as absorption centers. Most materials are composed of
materials which are selective in their absorption of light frequencies.
Thus they absorb only certain portions of the visible spectrum. The
frequencies of the spectrum which are not absorbed are either reflected
back or transmitted for our physical observation. In the visible portion
of the spectrum, this is what gives rise to color.
Absorption centers are largely responsible for the appearance of
specific wavelengths of visible light all around us. Moving from longer
(0.7 micrometer) to shorter (0.4 micrometer) wavelengths: red, orange,
yellow, green and blue (ROYGB) can all be identified by our senses in
the appearance of color by the selective absorption of specific light
wave frequencies (or wavelengths). Mechanisms of selective light wave
absorption include:
- Electronic: Transitions in electron energy levels within the atom (e.g., pigments). These transitions are typically in the ultraviolet (UV) and/or visible portions of the spectrum.
- Vibrational: Resonance in atomic/molecular vibrational modes. These transitions are typically in the infrared portion of the spectrum.
UV-Vis: Electronic transitions
In
electronic absorption, the frequency of the incoming light wave is at
or near the energy levels of the electrons within the atoms which
compose the substance. In this case, the electrons will absorb the
energy of the light wave and increase their energy state, often moving
outward from the nucleus of the atom into an outer shell or orbital.
The atoms that bind together to make the molecules of any particular substance contain a number of electrons (given by the atomic number Z in the periodic chart). Recall that all light waves are electromagnetic in origin. Thus they are affected strongly when coming into contact with negatively charged electrons in matter. When photons (individual packets of light energy) come in contact with the valence electrons of atom, one of several things can and will occur:
- A molecule absorbs the photon, some of the energy may be lost via luminescence, fluorescence and phosphorescence.
- A molecule absorbs the photon which results in reflection or scattering.
- A molecule cannot absorb the energy of the photon and the photon continues on its path. This results in transmission (provided no other absorption mechanisms are active).
Most of the time, it is a combination of the above that happens to
the light that hits an object. The states in different materials vary in
the range of energy that they can absorb. Most glasses, for example,
block ultraviolet (UV) light. What happens is the electrons in the glass
absorb the energy of the photons in the UV range while ignoring the
weaker energy of photons in the visible light spectrum. But there are
also existing special glass types, like special types of borosilicate glass or quartz that are UV-permeable and thus allow a high transmission of ultra violet light.
Thus, when a material is illuminated, individual photons of light can make the valence electrons of an atom transition to a higher electronic energy level.
The photon is destroyed in the process and the absorbed radiant energy
is transformed to electric potential energy. Several things can happen
then to the absorbed energy: it may be re-emitted by the electron as radiant energy
(in this case the overall effect is in fact a scattering of light),
dissipated to the rest of the material (i.e. transformed into heat), or the electron can be freed from the atom (as in the photoelectric and Compton effects).
Infrared: Bond stretching
Normal modes of vibration in a crystalline solid
The primary physical mechanism for storing mechanical energy of motion in condensed matter is through heat, or thermal energy.
Thermal energy manifests itself as energy of motion. Thus, heat is
motion at the atomic and molecular levels. The primary mode of motion in
crystalline substances is vibration. Any given atom will vibrate around some mean or average position within a crystalline structure, surrounded by its nearest neighbors. This vibration in two dimensions is equivalent to the oscillation of a clock’s pendulum. It swings back and forth symmetrically about some mean or average (vertical) position. Atomic and molecular vibrational frequencies may average on the order of 1012 cycles per second (Terahertz radiation).
When a light wave of a given frequency strikes a material with
particles having the same or (resonant) vibrational frequencies, then
those particles will absorb the energy of the light wave and transform
it into thermal energy of vibrational motion. Since different atoms and
molecules have different natural frequencies of vibration, they will
selectively absorb different frequencies (or portions of the spectrum)
of infrared light. Reflection and transmission of light waves occur
because the frequencies of the light waves do not match the natural
resonant frequencies of vibration of the objects. When infrared light of
these frequencies strikes an object, the energy is reflected or
transmitted.
If the object is transparent, then the light waves are passed on
to neighboring atoms through the bulk of the material and re-emitted on
the opposite side of the object. Such frequencies of light waves are
said to be transmitted.
Transparency in insulators
An
object may be not transparent either because it reflects the incoming
light or because it absorbs the incoming light. Almost all solids
reflect a part and absorb a part of the incoming light.
When light falls onto a block of metal, it encounters atoms that are tightly packed in a regular lattice and a "sea of electrons" moving randomly between the atoms.
In metals, most of these are non-bonding electrons (or free electrons)
as opposed to the bonding electrons typically found in covalently bonded
or ionically bonded non-metallic (insulating) solids. In a metallic
bond, any potential bonding electrons can easily be lost by the atoms in
a crystalline structure. The effect of this delocalization is simply to
exaggerate the effect of the "sea of electrons". As a result of these
electrons, most of the incoming light in metals is reflected back, which
is why we see a shiny metal surface.
Most insulators (or dielectric materials) are held together by ionic bonds. Thus, these materials do not have free conduction electrons,
and the bonding electrons reflect only a small fraction of the incident
wave. The remaining frequencies (or wavelengths) are free to propagate
(or be transmitted). This class of materials includes all ceramics and glasses.
If a dielectric material does not include light-absorbent
additive molecules (pigments, dyes, colorants), it is usually
transparent to the spectrum of visible light. Color centers
(or dye molecules, or "dopants") in a dielectric absorb a portion of
the incoming light. The remaining frequencies (or wavelengths) are free
to be reflected or transmitted. This is how colored glass is produced.
Most liquids and aqueous solutions are highly transparent. For
example, water, cooking oil, rubbing alcohol, air, and natural gas are
all clear. Absence of structural defects (voids, cracks, etc.) and
molecular structure of most liquids are chiefly responsible for their
excellent optical transmission. The ability of liquids to "heal"
internal defects via viscous flow is one of the reasons why some fibrous
materials (e.g., paper or fabric) increase their apparent transparency
when wetted. The liquid fills up numerous voids making the material more
structurally homogeneous.
Light scattering in an ideal defect-free crystalline (non-metallic) solid which provides no scattering centers for incoming light will be due primarily to any effects of anharmonicity within the ordered lattice. Light transmission will be highly directional due to the typical anisotropy of crystalline substances, which includes their symmetry group and Bravais lattice. For example, the seven different crystalline forms of quartz silica (silicon dioxide, SiO2) are all clear, transparent materials.
Optical waveguides
Propagation of light through a multi-mode optical fiber
A laser beam bouncing down an acrylic rod, illustrating the total internal reflection of light in a multimode optical fiber
Optically transparent materials focus on the response of a material
to incoming light waves of a range of wavelengths. Guided light wave
transmission via frequency selective waveguides involves the emerging
field of fiber optics and the ability of certain glassy compositions to act as a transmission medium for a range of frequencies simultaneously (multi-mode optical fiber) with little or no interference
between competing wavelengths or frequencies. This resonant mode of
energy and data transmission via electromagnetic (light) wave
propagation is relatively lossless.
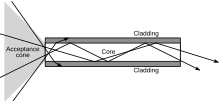
An optical fiber is a cylindrical dielectric waveguide that transmits light along its axis by the process of total internal reflection. The fiber consists of a core surrounded by a cladding layer. To confine the optical signal in the core, the refractive index of the core must be greater than that of the cladding. The refractive index is the parameter reflecting the speed of light
in a material. (Refractive index is the ratio of the speed of light in
vacuum to the speed of light in a given medium. The refractive index of
vacuum is therefore 1.) The larger the refractive index, the more slowly
light travels in that medium. Typical values for core and cladding of
an optical fiber are 1.48 and 1.46, respectively.
When light traveling in a dense medium hits a boundary at a steep
angle, the light will be completely reflected. This effect, called total internal reflection,
is used in optical fibers to confine light in the core. Light travels
along the fiber bouncing back and forth off of the boundary. Because the
light must strike the boundary with an angle greater than the critical angle, only light that enters the fiber within a certain range of angles will be propagated. This range of angles is called the acceptance cone
of the fiber. The size of this acceptance cone is a function of the
refractive index difference between the fiber's core and cladding. Optical waveguides are used as components in integrated optical circuits (e.g. combined with lasers or light-emitting diodes, LEDs) or as the transmission medium in local and long haul optical communication systems.
Mechanisms of attenuation
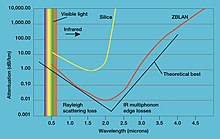
Light attenuation by ZBLAN and silica fibers
Attenuation in fiber optics,
also known as transmission loss, is the reduction in intensity of the
light beam (or signal) with respect to distance traveled through a
transmission medium. Attenuation coefficients in fiber optics usually
use units of dB/km through the medium due to the very high quality of
transparency of modern optical transmission media. The medium is usually
a fiber of silica glass that confines the incident light beam to the
inside. Attenuation is an important factor limiting the transmission of a
signal across large distances. In optical fibers the main attenuation
source is scattering from molecular level irregularities (Rayleigh scattering) due to structural disorder and compositional fluctuations of the glass structure. This same phenomenon is seen as one of the limiting factors in the transparency of infrared missile domes.
Further attenuation is caused by light absorbed by residual materials,
such as metals or water ions, within the fiber core and inner cladding.
Light leakage due to bending, splices, connectors, or other outside
forces are other factors resulting in attenuation.
As camouflage
Many animals of the open sea, like this Aurelia labiata jellyfish, are largely transparent.
Many marine animals that float near the surface are highly transparent, giving them almost perfect camouflage. However, transparency is difficult for bodies made of materials that have different refractive indices from seawater. Some marine animals such as jellyfish have gelatinous bodies, composed mainly of water; their thick mesogloea is acellular and highly transparent. This conveniently makes them buoyant,
but it also makes them large for their muscle mass, so they cannot swim
fast, making this form of camouflage a costly trade-off with mobility. Gelatinous planktonic
animals are between 50 and 90 percent transparent. A transparency of 50
percent is enough to make an animal invisible to a predator such as cod at a depth of 650 metres (2,130 ft); better transparency is required for invisibility
in shallower water, where the light is brighter and predators can see
better. For example, a cod can see prey that are 98 percent transparent
in optimal lighting in shallow water. Therefore, sufficient transparency
for camouflage is more easily achieved in deeper waters. For the same reason, transparency in air is even harder to achieve, but a partial example is found in the
glass frogs of the South American rain forest, which have translucent skin and pale greenish limbs. Several Central American species of clearwing (ithomiine) butterflies and many dragonflies and allied insects also have wings which are mostly transparent, a form of crypsis that provides some protection from predators.