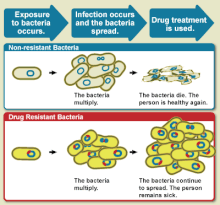
An illustrative diagram explaining drug resistance.
Drug resistance is the reduction in effectiveness of a medication such as an antimicrobial or an antineoplastic in treating a disease or condition. The term is used in the context of resistance that pathogens or cancers have "acquired", that is, resistance has evolved. Antimicrobial resistance and antineoplastic resistance challenge clinical care and drive research. When an organism is resistant to more than one drug, it is said to be multidrug-resistant.
The development of antibiotic resistance in particular stems from
the drugs targeting only specific bacterial molecules (almost always
proteins). Because the drug is so specific, any mutation in these
molecules will interfere with or negate its destructive effect,
resulting in antibiotic resistance. Furthermore, there is mounting concern over the abuse of antibiotics in the farming of livestock, which in the European Union alone accounts for three times the volume dispensed to humans – leading to development of super-resistant bacteria.
Bacteria are capable of not only altering the enzyme targeted by
antibiotics, but also by the use of enzymes to modify the antibiotic
itself and thus neutralize it. Examples of target-altering pathogens are
Staphylococcus aureus, vancomycin-resistant enterococci and macrolide-resistant Streptococcus, while examples of antibiotic-modifying microbes are Pseudomonas aeruginosa and aminoglycoside-resistant Acinetobacter baumannii.
In short, the lack of concerted effort by governments and the
pharmaceutical industry, together with the innate capacity of microbes
to develop resistance at a rate that outpaces development of new drugs,
suggests that existing strategies for developing viable, long-term
anti-microbial therapies are ultimately doomed to failure. Without
alternative strategies, the acquisition of drug resistance by pathogenic
microorganisms looms as possibly one of the most significant public
health threats facing humanity in the 21st century.
Types
Resistance
to chemicals is only one aspect of the problem, another being resistance
to physical factors such as temperature, pressure, sound, radiation and
magnetism, and not discussed in this article, but found at Physical factors affecting microbial life.
Drug, toxin, or chemical resistance is a consequence of evolution
and is a response to pressures imposed on any living organism.
Individual organisms vary in their sensitivity to the drug used and some
with greater fitness
may be capable of surviving drug treatment. Drug-resistant traits are
accordingly inherited by subsequent offspring, resulting in a population
that is more drug-resistant. Unless the drug used makes sexual
reproduction or cell-division or horizontal gene transfer impossible in the entire target population, resistance to the drug will inevitably follow. This can be seen in cancerous tumors where some cells may develop resistance to the drugs used in chemotherapy. Chemotherapy causes fibroblasts near tumors to produce large amounts of the protein WNT16B. This protein stimulates the growth of cancer cells which are drug-resistant. MicroRNAs have also been shown to affect acquired drug resistance in cancer cells and this can be used for therapeutic purposes. Malaria in 2012 has become a resurgent threat in South East Asia and sub-Saharan Africa, and drug-resistant strains of Plasmodium falciparum are posing massive problems for health authorities. Leprosy has shown an increasing resistance to dapsone.
A rapid process of sharing resistance exists among single-celled organisms, and is termed horizontal gene transfer in which there is a direct exchange of genes, particularly in the biofilm state. A similar asexual method is used by fungi and is called "parasexuality". Examples of drug-resistant strains are to be found in microorganisms such as bacteria and viruses, parasites both endo- and ecto-, plants, fungi, arthropods, mammals, birds, reptiles, fish, and amphibians.
In the domestic environment, drug-resistant strains of organism may arise from seemingly safe activities such as the use of bleach, tooth-brushing and mouthwashing, the use of antibiotics, disinfectants and detergents, shampoos, and soaps, particularly antibacterial soaps, hand-washing, usurface sprays, application of deodorants, sunblocks and any cosmetic or health-care product, insecticides, and dips.
The chemicals contained in these preparations, besides harming
beneficial organisms, may intentionally or inadvertently target
organisms that have the potential to develop resistance.
Mechanisms
The four main mechanisms by which microorganisms exhibit resistance to antimicrobials are:
- Drug inactivation or modification: e.g., enzymatic deactivation of Penicillin G in some penicillin-resistant bacteria through the production of β-lactamases.
- Alteration of target site: e.g., alteration of PBP — the binding target site of penicillins — in MRSA and other penicillin-resistant bacteria.
- Alteration of metabolic pathway: e.g., some sulfonamide-resistant bacteria do not require para-aminobenzoic acid (PABA), an important precursor for the synthesis of folic acid and nucleic acids in bacteria inhibited by sulfonamides. Instead, like mammalian cells, they turn to utilizing preformed folic acid.
- Reduced drug accumulation: by decreasing drug permeability and/or increasing active efflux (pumping out) of the drugs across the cell surface.
Mechanisms of Acquired Drug Resistance:
| Mechanism | Antimicrobial Agent | Drug Action | Mechanism of Resistance |
|---|---|---|---|
| Destroy drug | Aminoglycoside
Beta-lactam antibiotics (penicillin and cephalosporin)
Chloramphenicol |
Binds to 30S Ribosome subunit, inhibiting protein synthesis
Binds to penicillin-binding proteins, Inhibiting peptidoglycan synthesis
Bind to 50S ribosome subunit, inhibiting formation of peptide bonds |
Plasmid encode enzymes that chemically alter the drug (e.g., by acetylation or phosphorylation), thereby inactivating it.
Plasmid encode beta-lactamase, which open the beta-lactam ring, inactivating it.
Plasmid encode an enzyme that acetylate the drug, thereby inactivating it. |
| Alters drug target | Aminoglycosides
Beta-lactam antibiotics (penicillin and cephalosporin)
Erythromycin Quinolones Rifampin Trimethoprim |
Binds to 30S Ribosome subunit, inhibiting protein synthesis
Binds to penicillin-binding proteins, Inhibiting peptidoglycan synthesis
Bind to 50S ribosome subunit, inhibiting protein synthesis Binds to DNA topoisomerase, an enzyme essential for DNA synthesis Binds to the RNA polymerase; inhibiting initiation of RNA synthesis Inhibit the enzyme dihydrofolate reduces, blocking the folic acid pathway |
Bacteria make an altered 30S ribosomes that does not bind to the drug.
Bacteria make an altered penicillin-binding proteins, that do not bind to the drug.
Bacteria make a form of 50S ribosome that does not binds to the drug. Bacteria make an altered DNA topoisomerase that does not binds to the drug. Bacteria make an altered polymerase that does not binds to the drug. Bacteria make an altered enzyme that does not binds to the drug. |
| Inhibits drug entry or removes drug | Penicillin
Erythromycin
Tetracycline |
Binds to penicillin-binding proteins, Inhibiting peptidoglycan synthesis
Bind to 50S ribosome subunit, inhibiting protein synthesis
Binds to 30S Ribosome subunit, inhibiting protein synthesis by blocking tRNA |
Bacteria change shape of the outer membrane porin proteins, preventing drug from entering cell.
New membrane transport system prevent drug from entering cell.
New membrane transport system pumps drug out of cell. |
Metabolic cost
Biological cost is a measure of the increased energy metabolism required to achieve a function.
Drug resistance has a high metabolic price in pathogens for which this concept is relevant (bacteria,
endoparasites, and tumor cells.) In viruses, an equivalent "cost" is
genomic complexity. The high metabolic cost means that, in the absence
of antibiotics, a resistant pathogen will have decreased evolutionary
fitness as compared to susceptible pathogens.
This is one of the reasons drug resistance adaptations are rarely seen
in environments where antibiotics are absent. However, in the presence
of antibiotics, the survival advantage conferred off-sets the high
metabolic cost and allows resistant strains to proliferate.
Treatment
In humans, the gene ABCB1 encodes MDR1(p-glycoprotein) which is a key transporter of medications on the cellular level. If MDR1 is overexpressed, drug resistance increases. Therefore, ABCB1 levels can be monitored.
In patients with high levels of ABCB1 expression, the use of secondary
treatments, like metformin, have been used in conjunction with the
primary drug treatment with some success.
For antibiotic resistance,
which represents a widespread problem nowadays, drugs designed to block
the mechanisms of bacterial antibiotic resistance are used. For
example, bacterial resistance against beta-lactam antibiotics (such as penicillin and cephalosporins) can be circumvented by using antibiotics such as nafcillin that are not susceptible to destruction by certain beta-lactamases (the group of enzymes responsible for breaking down beta-lactams).
Beta-lactam bacterial resistance can also be dealt with by
administering beta-lactam antibiotics with drugs that block
beta-lactamases such as clavulanic acid so that the antibiotics can work without getting destroyed by the bacteria first. Recently, researchers have recognized the need for new drugs that inhibit bacterial efflux pumps, which cause resistance to multiple antibiotics such as beta-lactams, quinolones, chloramphenicol, and trimethoprim by sending molecules of those antibiotics out of the bacterial cell.
Sometimes a combination of different classes of antibiotics may be used
synergistically; that is, they work together to effectively fight
bacteria that may be resistant to one of the antibiotics alone.
Destruction of the resistant bacteria can also be achieved by phage therapy, in which a specific bacteriophage (virus that kills bacteria) is used.
There is research being done using antimicrobial peptides. In the future, there is a possibility that they might replace novel antibiotics.