 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌdɒksəˈruːbɪsɪn/ |
| Trade names | Adriamycin, Caelyx, Myocet, others |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682221 |
| License data |
|
| Pregnancy category | |
| Routes of administration | intravenous, intravesical |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 5% (by mouth) |
| Protein binding | 75% |
| Metabolism | Liver |
| Elimination half-life | Triphasic; 12 minutes, 3.3 hours, 30 hours. Mean: 1–3 hours |
| Excretion | Urine (5–12%), faeces (40–50%) |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.041.344 |
| Chemical and physical data | |
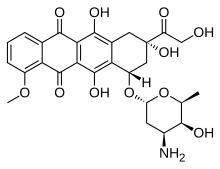
| Formula | C27H29NO11 |
| Molar mass | 543.525 g·mol−1 |
| 3D model (JSmol) | |
Doxorubicin, sold under the brand name Adriamycin among others, is a chemotherapy medication used to treat cancer. This includes breast cancer, bladder cancer, Kaposi's sarcoma, lymphoma, and acute lymphocytic leukemia. It is often used together with other chemotherapy agents. Doxorubicin is given by injection into a vein.
Common side effects include hair loss, bone marrow suppression, vomiting, rash, and inflammation of the mouth. Other serious side effects may include allergic reactions such as anaphylaxis, heart damage, tissue damage at the site of injection, radiation recall, and treatment-related leukemia. People often experience red discoloration of the urine for a few days. Doxorubicin is in the anthracycline and antitumor antibiotic family of medications. It works in part by interfering with the function of DNA.
Doxorubicin was approved for medical use in the United States in 1974. It is on the World Health Organization's List of Essential Medicines, the safest and most effective medicines needed in a health system. The wholesale cost in the developing world is about US$3.88–32.79 per 50 mg vial. In the United Kingdom this amount costs the NHS about £100.12. Versions that are pegylated and in liposomes are also available; however, are more expensive. Doxorubicin was originally made from the bacteria Streptomyces peucetius.
Medical use
Doxorubicin is commonly used to treat some leukemias and Hodgkin's lymphoma, as well as cancers of the bladder, breast, stomach, lung, ovaries, thyroid, soft tissue sarcoma, multiple myeloma, and others. Commonly used doxorubicin-containing regimens are AC (Adriamycin, cyclophosphamide), TAC (taxotere, AC), ABVD (Adriamycin, bleomycin, vinblastine, dacarbazine), BEACOPP, CHOP (cyclophosphamide, hydroxydaunorubicin, vincristine, prednisone) and FAC (5-fluorouracil, adriamycin, cyclophosphamide).
Doxil (see below) is used primarily for the treatment of ovarian cancer where the disease has progressed or recurred after platinum-based chemotherapy, or for the treatment of AIDS-related Kaposi's sarcoma.
Liposomal form
There is a pegylated (polyethylene glycol coated) liposome-encapsulated form of doxorubicin, sold as Doxil. It was developed to treat Kaposi's sarcoma, an AIDS-related
cancer that causes lesions to grow under the skin, in the lining of the
mouth, nose and throat, or in other organs. The polyethylene glycol coating results in preferential concentration of doxorubicin in the skin. However, this also results in a side effect called palmar plantar erythrodysesthesia (PPE), more commonly known as hand-foot syndrome.
Following administration of this form of doxorubicin, small
amounts of the drug can leak from capillaries in the palms of the hands
and soles of the feet. The result of this leakage is redness,
tenderness, and peeling of the skin that can be uncomfortable and even
painful. In clinical testing at 50 mg/m2 dosing every 4
weeks, half of people developed hand-foot syndrome. The rate of this
side effect limits the dose of this formulation that can be given as
compared with plain doxorubicin in the same treatment regimen, thereby
limiting potential substitution. Substitution would be desirable because
liposome-encapsulated doxorubicin is less cardiotoxic than
unencapsulated doxorubicin. This form is also approved by the FDA for treatment of ovarian cancer and multiple myeloma.
A non-pegylated liposomal doxorubicin, called Myocet, is approved
in Europe and Canada for treatment of metastatic breast cancer in
combination with cyclophosphamide,
but has not been approved by the FDA for use in the United States.
Unlike Doxil, the Myocet liposome does not have a polyethylene glycol
coating, and therefore does not result in the same rate of hand-foot
syndrome. The minimization of this side effect may allow for one-for-one
(1:1) substitution with doxorubicin in the same treatment regimen,
thereby improving safety with no loss of efficacy. Like Doxil, the
liposomal encapsulation of the doxorubicin limits the cardiotoxicity. In
theory, by limiting the cardiotoxicity of doxorubicin through liposomal
encapsulation, it can be used safely in concurrent combination with
other cardiotoxic chemotherapy drugs, such as trastuzumab. There is an
FDA black box warning that trastuzumab cannot be used in concurrent
combination with doxorubicin, only in sequential combination. Though
concurrent combination of trastuzumab and doxorubicin in clinical
studies found superior tumor response, the combination resulted in
unacceptable cardiotoxicity, including risk of cardiac failure
manifesting as congestive heart failure
(CHF). Published phase II study results have shown that Myocet,
trastuzumab, and paclitaxel can safely be used concurrently without the
cardiac risk, as measured by reduction in LVEF
function, while still achieving superior tumor response. This finding
is the basis for the ongoing phase III trial for FDA approval.
Side effects
Cardiotoxicity
The most dangerous side effect of doxorubicin is dilated cardiomyopathy, leading to congestive heart failure.
The rate of cardiomyopathy is dependent on its cumulative dose, with an
incidence about 4% when the dose of doxorubicin is 500–550 mg/m2, 18% when the dose is 551–600 mg/m2 and 36% when the dose exceeds 600 mg/m2. There are several ways in which doxorubicin is believed to cause cardiomyopathy, including oxidative stress, downregulation of genes for contractile proteins, and p53-mediated apoptosis.
Doxorubicin-induced cardiomyopathy typically results in dilated cardiomyopathy, with all four cardiac chambers being enlarged. This results in both systolic and diastolic dysfunction. Eventually, heart failure can result, which carries a 50% mortality rate. There is no effective treatment against established cardiomyopathy caused by the drug as of 2010. The drug dexrazoxane may be used to decrease the risk of doxorubicin's cardiotoxicity in certain cases.
Other
Another common and potentially fatal complication of doxorubicin is typhlitis, an acute life-threatening infection of the bowel. Additionally, some people may develop PPE, characterized by skin eruptions on the palms of the hand or soles of the feet, swelling, pain, and erythema. Due to these side effects and its red color, doxorubicin has earned the nickname "red devil" or "red death."
Chemotherapy can cause reactivation of hepatitis B, and doxorubicin-containing regimens are no exception.
Doxorubicin and several chemotherapeutic drugs (including
cyclophosphamide) cause dyspigmentation. Other groups of drugs that
cause this problem include antimalarials, amiodarone, heavy metals (but not iron), tetracyclines, and antipsychotics.
Biosynthesis
Doxorubicin (DXR) is a 14-hydroxylated version of daunorubicin, the immediate precursor of DXR in its biosynthetic pathway.
Daunorubicin is more abundantly found as a natural product because it is produced by a number of different wild type strains of Streptomyces. In contrast, only one known non-wild type species, Streptomyces peucetius subspecies cesius ATCC 27952, was initially found to be capable of producing the more widely used doxorubicin. This strain was created by Arcamone et al. in 1969 by mutating a strain producing daunorubicin, but not DXR, at least in detectable quantities. Subsequently, Hutchinson's group showed that under special environmental conditions, or by the introduction of genetic modifications, other strains of Streptomyces can produce doxorubicin. His group also cloned many of the genes
required for DXR production, although not all of them have been fully
characterized. In 1996, Strohl's group discovered, isolated and
characterized dox A, the gene encoding the enzyme that converts daunorubicin into DXR.
By 1999, they produced recombinant dox A, a cytochrome P450 oxidase, and found that it catalyzes multiple steps in DXR biosynthesis, including steps leading to daunorubicin. This was significant because it became clear that all daunorubicin-producing strains have the necessary genes
to produce DXR, the much more therapeutically important of the two.
Hutchinson's group went on to develop methods to improve the yield of
DXR, from the fermentation process used in its commercial production, not only by introducing dox A encoding plasmids, but also by introducing mutations to deactivate enzymes that shunt DXR precursors to less useful products, for example baumycin-like glycosides. Some triple mutants, that also over-expressed
dox A, were able to double the yield of DXR. This is of more than
academic interest, because at that time DXR cost about $1.37 million per
kg and current production in 1999 was 225 kg per annum.
More efficient production techniques have brought the price down to $1.1 million per kg for the nonliposomal formulation. Although DXR can be produced semi-synthetically from daunorubicin, the process involves electrophilic bromination and multiple steps, and the yield is poor. Since daunorubicin is produced by fermentation, it would be ideal if the bacteria could complete DXR synthesis more effectively.
Mechanism of action
Doxorubicin interacts with DNA by intercalation and inhibition of macromolecular biosynthesis. This inhibits the progression of topoisomerase II, an enzyme which relaxes supercoils in DNA for transcription.
Doxorubicin stabilizes the topoisomerase II complex after it has broken
the DNA chain for replication, preventing the DNA double helix from
being resealed and thereby stopping the process of replication. It may also increase quinone type free radical production, hence contributing to its cytotoxicity.
The planar aromatic chromophore portion of the molecule
intercalates between two base pairs of the DNA, while the six-membered
daunosamine sugar sits in the minor groove and interacts with flanking
base pairs immediately adjacent to the intercalation site, as evidenced
by several crystal structures.
By intercalation, doxorubicin can also induce histone eviction from transcriptionally active chromatin. As a result, DNA damage response, epigenome and transcriptome are deregulated in doxorubicin-exposed cells.
History
In the 1950s, an Italian research company, Farmitalia
Research Laboratories, began an organized effort to find anticancer
compounds from soil-based microbes. A soil sample was isolated from the
area surrounding the Castel del Monte, a 13th-century castle. A new strain of Streptomyces peucetius,
which produced a red pigment, was isolated, and an antibiotic from this
bacterium was effective against tumors in mice. Since a group of French
researchers discovered the same compound at about the same time, the
two teams named the compound daunorubicin, combining the name Dauni, a pre-Roman tribe that occupied the area of Italy where the compound was isolated, with the French word for ruby, rubis, describing the color.
Clinical trials began in the 1960s, and the drug was successful in
treating acute leukemia and lymphoma. However, by 1967, it was
recognized that daunorubicin could lead to fatal cardiac toxicity.
Researchers at Farmitalia soon discovered that changes in
biological activity could be made by minor changes in the structure of
the compound. A strain of Streptomyces was mutated using N-nitroso-N-methyl
urethane, and this new strain produced a different, red-colored
antibiotic. They named this new compound Adriamycin, after the Adriatic Sea, and the name was later changed to doxorubicin to conform to the established naming convention.
Doxorubicin showed better activity than daunorubicin against mouse
tumors, and especially solid tumors. It also showed a higher therapeutic index, yet the cardiotoxicity remained.
Doxorubicin and daunorubicin together can be thought of as prototype compounds for the anthracyclines.
Subsequent research has led to many other anthracycline antibiotics, or
analogs, and there are now over 2,000 known analogs of doxorubicin. By
1991, 553 of them had been evaluated in the screening program at the National Cancer Institute (NCI). In 2016 GPX-150 was granted orphan drug designation by US FDA.
Society and culture
Names
It is also known as hydroxydaunorubicin and hydroxydaunomycin.
It is sold under a number of different brand names, including Adriamycin PFS, Adriamycin RDF, or Rubex.
Formulations
Doxorubicin
is photosensitive, and containers are often covered by an aluminum bag
and/or brown wax paper to prevent light from affecting it. Doxorubicin is also available in liposome-encapsulated forms as Doxil (pegylated form), Myocet (nonpegylated form), and Caelyx, although these forms must also be given by intravenous injection.
Shortage
Between 2011 and March 2014, Doxil was in limited supply.
In 2011, Doxil became available only in very limited supply due to
production problems with the third-party manufacturer. Johnson &
Johnson (JNJ), through its subsidiary Janssen Products, LP, had been
receiving its Doxil supply from contract manufacturer Ben Venue
Laboratories (located in Bedford, Ohio), a unit of Boehringer Ingelheim GmbH of Germany. The problems began when Ben Venue temporarily shut down their manufacturing facility due to quality control issues.
In February 2012, to address the Doxil shortage, the US Food and
Drug Administration (FDA) allowed for the temporary importation of
Lipodox, which contains the same active ingredient as Doxil and is made
by Sun Pharma Global FZE (Sun), a subsidiary of India's Sun
Pharmaceutical Industries Ltd.
The agency said it intends to continue allowing the importation of
Lipodox until Sun has made enough generic Doxil to meet demand.
The FDA approved the first generic version of Doxil, made by Sun,
in February 2013. It will be available in 20 milligram and 50
milligram vials.
Research
Combination therapy experiments with sirolimus (rapamycin) and doxorubicin have shown promise in treating Akt-positive lymphomas in mice.
Recent animal research coupling a murine monoclonal antibody with doxorubicin has created an immunoconjugate that was able to eliminate HIV-1 infection in mice. Current treatment with antiretroviral therapy
(ART) still leaves pockets of HIV within the host. The immunoconjugate
could potentially provide a complementary treatment to ART to eradicate
antigen-expressing T cells.
Antimalarial activity
There
is some evidence for antimalarial activity for doxorubicin and similar
compounds. In 2009, a compound similar in structure to doxorubicin was
found to inhibit plasmepsin II, an enzyme unique to the malarial parasite Plasmodium falciparum. The pharmaceutical company GlaxoSmithKline (GSK) later identified doxorubicin in a set of compounds that inhibit parasite growth
Fluorescence
Doxorubicin
is also known to be fluorescent. This has often been used to
characterize doxorubicin concentrations, and has opened the possibility
of using the molecule as a theranostic
agent. However, there are significant limitations, as doxorubicin's
fluorescence spectrum is known to depend on a variety of factors,
including the pH of the environment, solvent dielectric constant
and others. Doxorubicin fluorescence is quenched by binding to DNA, and
shielded by micelle encapsulation. It is also known to self-quench at
high concentrations. In contrast, histone binding amplifies
fluorescence.

