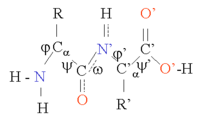Constituent amino-acids can be analyzed to predict secondary, tertiary and quaternary protein structure.
Protein structure prediction is the inference of the three-dimensional structure of a protein from its amino acid sequence—that is, the prediction of its folding and its secondary and tertiary structure from its primary structure. Structure prediction is fundamentally different from the inverse problem of protein design. Protein structure prediction is one of the most important goals pursued by bioinformatics and theoretical chemistry; it is highly important in medicine (for example, in drug design) and biotechnology (for example, in the design of novel enzymes). Every two years, the performance of current methods is assessed in the CASP
experiment (Critical Assessment of Techniques for Protein Structure
Prediction). A continuous evaluation of protein structure prediction web
servers is performed by the community project CAMEO3D.
Protein structure and terminology
Proteins
are chains of amino acids joined together by peptide bonds. Many
conformations of this chain are possible due to the rotation of the
chain about each Cα atom. It is these conformational changes that are
responsible for differences in the three dimensional structure of
proteins. Each amino acid in the chain is polar, i.e. it has separated
positive and negative charged regions with a free carbonyl group,
which can act as hydrogen bond acceptor and an NH group, which can act
as hydrogen bond donor. These groups can therefore interact in the
protein structure. The 20 amino acids can be classified according to
the chemistry of the side chain which also plays an important structural
role. Glycine
takes on a special position, as it has the smallest side chain, only
one hydrogen atom, and therefore can increase the local flexibility in
the protein structure. Cysteine on the other hand can react with another cysteine residue and thereby form a cross link stabilizing the whole structure.
The protein structure can be considered as a sequence of
secondary structure elements, such as α helices and β sheets, which
together constitute the overall three-dimensional configuration of the
protein chain. In these secondary structures regular patterns of H bonds
are formed between neighboring amino acids, and the amino acids have
similar Φ and Ψ angles.
Bond angles for ψ and ω
The formation of these structures neutralizes the polar groups on
each amino acid. The secondary structures are tightly packed in the
protein core in a hydrophobic environment. Each amino acid side group
has a limited volume to occupy and a limited number of possible
interactions with other nearby side chains, a situation that must be
taken into account in molecular modeling and alignments.
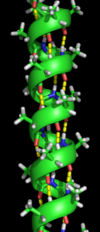
α Helix
The α helix is the most abundant type of secondary structure in
proteins. The α helix has 3.6 amino acids per turn with an H bond formed
between every fourth residue; the average length is 10 amino acids (3
turns) or 10 Å but varies from 5 to 40 (1.5 to 11 turns). The alignment
of the H bonds creates a dipole moment for the helix with a resulting
partial positive charge at the amino end of the helix. Because this
region has free NH2 groups, it will interact with
negatively charged groups such as phosphates. The most common location
of α helices is at the surface of protein cores, where they provide an
interface with the aqueous environment. The inner-facing side of the
helix tends to have hydrophobic amino acids and the outer-facing side
hydrophilic amino acids. Thus, every third of four amino acids along the
chain will tend to be hydrophobic, a pattern that can be quite readily
detected. In the leucine zipper motif, a repeating pattern of leucines
on the facing sides of two adjacent helices is highly predictive of the
motif. A helical-wheel plot can be used to show this repeated pattern.
Other α helices buried in the protein core or in cellular
membranes have a higher and more regular distribution of
hydrophobic amino acids, and are highly predictive of such structures.
Helices exposed on the surface have a lower proportion of hydrophobic
amino acids. Amino acid content can be predictive of an α -helical
region. Regions richer in alanine (A), glutamic acid (E), leucine (L), and methionine (M) and poorer in proline (P), glycine (G), tyrosine (Y), and serine
(S) tend to form an α helix. Proline destabilizes or breaks an α
helix but can be present in longer helices, forming a bend.
An alpha-helix with hydrogen bonds (yellow dots)
β sheet
β sheets are formed by H bonds between an average of 5–10 consecutive
amino acids in one portion of the chain with another 5–10 farther down
the chain. The interacting regions may be adjacent, with a short loop in
between, or far apart, with other structures in between. Every chain
may run in the same direction to form a parallel sheet, every other
chain may run in the reverse chemical direction to form an anti parallel
sheet, or the chains may be parallel and anti parallel to form a mixed
sheet. The pattern of H bonding is different in the parallel and anti
parallel configurations. Each amino acid in the interior strands of the
sheet forms two H bonds with neighboring amino acids, whereas each amino
acid on the outside strands forms only one bond with an interior
strand. Looking across the sheet at right angles to the strands, more
distant strands are rotated slightly counterclockwise to form a
left-handed twist. The Cα atoms alternate above and below the sheet in a
pleated structure, and the R side groups of the amino acids alternate
above and below the pleats. The Φ and Ψ angles of the amino acids in
sheets vary considerably in one region of the Ramachandran plot.
It is more difficult to predict the location of β sheets than of α
helices. The situation improves somewhat when the amino acid variation
in multiple sequence alignments is taken into account.
Loop
Loops are
regions of a protein chain that are 1) between α helices and β
sheets, 2) of various lengths and three-dimensional configurations, and
3) on the surface of the structure.
Hairpin loops that represent a complete turn in the polypeptide
chain joining two antiparallel β strands may be as short as two amino
acids in length. Loops interact with the surrounding aqueous environment
and other proteins. Because amino acids in loops are not constrained by
space and environment as are amino acids in the core region, and do not
have an effect on the arrangement of secondary structures in the core,
more substitutions, insertions, and deletions may occur. Thus, in a
sequence alignment, the presence of these features may be an indication
of a loop. The positions of introns in genomic DNA sometimes correspond to the locations of loops in the encoded protein.
Loops also tend to have charged and polar amino acids and are
frequently a component of active sites. A detailed examination of loop
structures has shown that they fall into distinct families.
Coils
A region of secondary structure that is not a α helix, a β sheet, or a recognizable turn is commonly referred to as a coil.
Protein classification
Proteins
may be classified according to both structural and sequence similarity.
For structural classification, the sizes and spatial arrangements of
secondary structures described in the above paragraph are compared in
known three-dimensional structures. Classification based on sequence
similarity was historically the first to be used. Initially, similarity
based on alignments of whole sequences was performed. Later, proteins
were classified on the basis of the occurrence of conserved amino acid
patterns. Databases
that classify proteins by one or more of these schemes are available.
In considering protein classification schemes, it is important to keep
several observations in mind. First, two entirely different protein
sequences from different evolutionary origins may fold into a similar
structure. Conversely, the sequence of an ancient gene for a given
structure may have diverged considerably in different species while at
the same time maintaining the same basic structural features.
Recognizing any remaining sequence similarity in such cases may be a
very difficult task. Second, two proteins that share a significant
degree of sequence similarity either with each other or with a third
sequence also share an evolutionary origin and should share some
structural features also. However, gene duplication and genetic
rearrangements during evolution may give rise to new gene copies, which
can then evolve into proteins with new function and structure.
Terms used for classifying protein structures and sequences
The
more commonly used terms for evolutionary and structural relationships
among proteins are listed below. Many additional terms are used for
various kinds of structural features found in proteins. Descriptions of
such terms may be found at the CATH Web site, the Structural Classification of Proteins (SCOP) Web site, and a Glaxo-Wellcome tutorial on the Swiss bioinformatics Expasy Web site.
Active site
is a localized combination of amino acid side groups within the
tertiary (three-dimensional) or quaternary (protein subunit) structure
that can interact with a chemically specific substrate and that provides
the protein with biological activity. Proteins of very different amino
acid sequences may fold into a structure that produces the same active
site.
Architecture is the relative orientations of secondary structures
in a three-dimensional structure without regard to whether or not they
share a similar loop structure.
Fold is a type of architecture that also has a conserved loop structure.
Blocks is a conserved amino acid sequence pattern in a family of
proteins. The pattern includes a series of possible matches at each
position in the represented sequences, but there are not any inserted or
deleted positions in the pattern or in the sequences. By way of
contrast, sequence profiles are a type of scoring matrix that represents
a similar set of patterns that includes insertions and deletions.
Class is a term used to classify protein domains according to their secondary structural content and organization. Four classes
were originally recognized by Levitt and Chothia (1976), and several
others have been added in the SCOP database. Three classes are given in
the CATH database: mainly-α, mainly-β, and α–β, with the α–β class
including both alternating α/β and α+β structures.
Core is the portion of a folded protein molecule that comprises
the hydrophobic interior of α-helices and β-sheets. The compact
structure brings together side groups of amino acids into close enough
proximity so that they can interact. When comparing protein structures,
as in the SCOP database, core is the region common to most of the
structures that share a common fold or that are in the same superfamily.
In structure prediction, core is sometimes defined as the arrangement
of secondary structures that is likely to be conserved during
evolutionary change.
Domain
(sequence context) is a segment of a polypeptide chain that can fold
into a three-dimensional structure irrespective of the presence of other
segments of the chain. The separate domains of a given protein may
interact extensively or may be joined only by a length of polypeptide
chain. A protein with several domains may use these domains for
functional interactions with different molecules.
Family
(sequence context) is a group of proteins of similar biochemical
function that are more than 50% identical when aligned. This same cutoff
is still used by the Protein Information Resource
(PIR). A protein family comprises proteins with the same function in
different organisms (orthologous sequences) but may also include
proteins in the same organism (paralogous sequences) derived from gene
duplication and rearrangements. If a multiple sequence alignment of a
protein family reveals a common level of similarity throughout the
lengths of the proteins, PIR refers to the family as a homeomorphic
family. The aligned region is referred to as a homeomorphic domain, and
this region may comprise several smaller homology domains that are
shared with other families. Families may be further subdivided into
subfamilies or grouped into superfamilies based on respective higher or
lower levels of sequence similarity. The SCOP database reports 1296
families and the CATH database (version 1.7 beta), reports 1846
families.
When the sequences of proteins with the same function are
examined in greater detail, some are found to share high sequence
similarity. They are obviously members of the same family by the above
criteria. However, others are found that have very little, or even
insignificant, sequence similarity with other family members. In such
cases, the family relationship between two distant family members A and C
can often be demonstrated by finding an additional family member B that
shares significant similarity with both A and C. Thus, B provides a
connecting link between A and C. Another approach is to examine distant
alignments for highly conserved matches.
At a level of identity of 50%, proteins are likely to have the
same three-dimensional structure, and the identical atoms in the
sequence alignment will also superimpose within approximately 1 Å in the
structural model. Thus, if the structure of one member of a family is
known, a reliable prediction may be made for a second member of the
family, and the higher the identity level, the more reliable the
prediction. Protein structural modeling can be performed by examining
how well the amino acid substitutions fit into the core of the
three-dimensional structure.
Family (structural context) is as used in the FSSP database (Families of structurally similar proteins)
and the DALI/FSSP Web site, two structures that have a significant
level of structural similarity but not necessarily significant sequence
similarity.
Fold is similar to structural motif, includes a larger
combination of secondary structural units in the same configuration.
Thus, proteins sharing the same fold have the same combination of
secondary structures that are connected by similar loops. An example is
the Rossman fold comprising several alternating α helices and parallel
β strands. In the SCOP, CATH, and FSSP databases, the known protein
structures have been classified into hierarchical levels of structural
complexity with the fold as a basic level of classification.
Homologous domain (sequence context) is an extended sequence
pattern, generally found by sequence alignment methods, that indicates a
common evolutionary origin among the aligned sequences. A homology
domain is generally longer than motifs. The domain may include all of a
given protein sequence or only a portion of the sequence. Some domains
are complex and made up of several smaller homology domains that became
joined to form a larger one during evolution. A domain that covers an
entire sequence is called the homeomorphic domain by PIR (Protein Information Resource).
Module is a region of conserved amino acid patterns comprising
one or more motifs and considered to be a fundamental unit of structure
or function. The presence of a module has also been used to classify
proteins into families.
Motif (sequence context) is a conserved pattern of amino acids that is found in two or more proteins. In the Prosite
catalog, a motif is an amino acid pattern that is found in a group of
proteins that have a similar biochemical activity, and that often is
near the active site of the protein. Examples of sequence motif
databases are the Prosite catalog and the Stanford Motifs Database.
Motif (structural context) is a combination of several secondary
structural elements produced by the folding of adjacent sections of the
polypeptide chain into a specific three-dimensional configuration. An
example is the helix-loop-helix motif. Structural motifs are also
referred to as supersecondary structures and folds.
Position-specific scoring matrix (sequence context, also known as
weight or scoring matrix) is represents a conserved region in a
multiple sequence alignment with no gaps. Each matrix column represents
the variation found in one column of the multiple sequence alignment.
Position-specific scoring matrix—3D (structural context)
represents the amino acid variation found in an alignment of proteins
that fall into the same structural class. Matrix columns represent the
amino acid variation found at one amino acid position in the aligned
structures.
Primary structure
is the linear amino acid sequence of a protein, which chemically is a
polypeptide chain composed of amino acids joined by peptide bonds.
Profile (sequence context) is a scoring matrix that represents a
multiple sequence alignment of a protein family. The profile is usually
obtained from a well-conserved region in a multiple sequence alignment.
The profile is in the form of a matrix with each column representing a
position in the alignment and each row one of the amino acids. Matrix
values give the likelihood of each amino acid at the corresponding
position in the alignment. The profile is moved along the target
sequence to locate the best scoring regions by a dynamic programming
algorithm. Gaps are allowed during matching and a gap penalty is
included in this case as a negative score when no amino acid is matched.
A sequence profile may also be represented by a hidden Markov model, referred to as a profile HMM.
Profile (structural context) is a scoring matrix that represents
which amino acids should fit well and which should fit poorly at
sequential positions in a known protein structure. Profile columns
represent sequential positions in the structure, and profile rows
represent the 20 amino acids. As with a sequence profile, the structural
profile is moved along a target sequence to find the highest possible
alignment score by a dynamic programming algorithm. Gaps may be included
and receive a penalty. The resulting score provides an indication as to
whether or not the target protein might adopt such a structure.
Quaternary structure is the three-dimensional configuration of a protein molecule comprising several independent polypeptide chains.
Secondary structure
is the interactions that occur between the C, O, and NH groups on amino
acids in a polypeptide chain to form α-helices, β-sheets, turns, loops,
and other forms, and that facilitate the folding into a
three-dimensional structure.
Superfamily
is a group of protein families of the same or different lengths that
are related by distant yet detectable sequence similarity. Members of a
given superfamily
thus have a common evolutionary origin. Originally, Dayhoff defined the
cutoff for superfamily status as being the chance that the sequences
are not related of 10 6, on the basis of an alignment score (Dayhoff et
al. 1978). Proteins with few identities in an alignment of the sequences
but with a convincingly common number of structural and functional
features are placed in the same superfamily. At the level of
three-dimensional structure, superfamily proteins will share common
structural features such as a common fold, but there may also be
differences in the number and arrangement of secondary structures. The
PIR resource uses the term homeomorphic superfamilies to refer to
superfamilies that are composed of sequences that can be aligned from
end to end, representing a sharing of single sequence homology domain, a
region of similarity that extends throughout the alignment. This domain
may also comprise smaller homology domains that are shared with other
protein families and superfamilies. Although a given protein sequence
may contain domains found in several superfamilies, thus indicating a
complex evolutionary history, sequences will be assigned to only one
homeomorphic superfamily based on the presence of similarity throughout
a multiple sequence alignment. The superfamily alignment may also
include regions that do not align either within or at the ends of the
alignment. In contrast, sequences in the same family align well
throughout the alignment.
Supersecondary structure
is a term with similar meaning to a structural motif. Tertiary
structure is the three-dimensional or globular structure formed by the
packing together or folding of secondary structures of a polypeptide
chain.
Secondary structure
Secondary structure prediction is a set of techniques in bioinformatics that aim to predict the local secondary structures of proteins based only on knowledge of their amino acid sequence. For proteins, a prediction consists of assigning regions of the amino acid sequence as likely alpha helices, beta strands (often noted as "extended" conformations), or turns. The success of a prediction is determined by comparing it to the results of the DSSP algorithm (or similar e.g. STRIDE) applied to the crystal structure of the protein. Specialized algorithms have been developed for the detection of specific well-defined patterns such as transmembrane helices and coiled coils in proteins.
The best modern methods of secondary structure prediction in proteins reach about 80% accuracy; this high accuracy allows the use of the predictions as feature improving fold recognition and ab initio protein structure prediction, classification of structural motifs, and refinement of sequence alignments. The accuracy of current protein secondary structure prediction methods is assessed in weekly benchmarks such as LiveBench and EVA.
Background
Early methods of secondary structure prediction, introduced in the 1960s and early 1970s, focused on identifying likely alpha helices and were based mainly on helix-coil transition models.
Significantly more accurate predictions that included beta sheets were
introduced in the 1970s and relied on statistical assessments based on
probability parameters derived from known solved structures. These
methods, applied to a single sequence, are typically at most about
60-65% accurate, and often underpredict beta sheets. The evolutionary conservation of secondary structures can be exploited by simultaneously assessing many homologous sequences in a multiple sequence alignment,
by calculating the net secondary structure propensity of an aligned
column of amino acids. In concert with larger databases of known protein
structures and modern machine learning methods such as neural nets and support vector machines, these methods can achieve up to 80% overall accuracy in globular proteins. The theoretical upper limit of accuracy is around 90%,
partly due to idiosyncrasies in DSSP assignment near the ends of
secondary structures, where local conformations vary under native
conditions but may be forced to assume a single conformation in crystals
due to packing constraints. Limitations are also imposed by secondary
structure prediction's inability to account for tertiary structure;
for example, a sequence predicted as a likely helix may still be able
to adopt a beta-strand conformation if it is located within a beta-sheet
region of the protein and its side chains pack well with their
neighbors. Dramatic conformational changes related to the protein's
function or environment can also alter local secondary structure.
Historical perspective
To date, over 20 different secondary structure prediction methods have been developed. One of the first algorithms was Chou-Fasman method,
which relies predominantly on probability parameters determined from
relative frequencies of each amino acid's appearance in each type of
secondary structure.
The original Chou-Fasman parameters, determined from the small sample
of structures solved in the mid-1970s, produce poor results compared to
modern methods, though the parameterization has been updated since it
was first published. The Chou-Fasman method is roughly 50-60% accurate
in predicting secondary structures.
The next notable program was the GOR method, named for the three scientists who developed it — Garnier, Osguthorpe, and Robson, is an information theory-based method. It uses the more powerful probabilistic technique of Bayesian inference.
The GOR method takes into account not only the probability of each
amino acid having a particular secondary structure, but also the conditional probability
of the amino acid assuming each structure given the contributions of
its neighbors (it does not assume that the neighbors have that same
structure). The approach is both more sensitive and more accurate than
that of Chou and Fasman because amino acid structural propensities are
only strong for a small number of amino acids such as proline and glycine.
Weak contributions from each of many neighbors can add up to strong
effects overall. The original GOR method was roughly 65% accurate and is
dramatically more successful in predicting alpha helices than beta
sheets, which it frequently mispredicted as loops or disorganized
regions.
Another big step forward, was using machine learning methods. First artificial neural networks
methods were used. As a training sets they use solved structures to
identify common sequence motifs associated with particular arrangements
of secondary structures. These methods are over 70% accurate in their
predictions, although beta strands are still often underpredicted due to
the lack of three-dimensional structural information that would allow
assessment of hydrogen bonding patterns that can promote formation of the extended conformation required for the presence of a complete beta sheet. PSIPRED and JPRED are some of the most known programs based on neural networks for protein secondary structure prediction. Next, support vector machines have proven particularly useful for predicting the locations of turns, which are difficult to identify with statistical methods.
Extensions of machine learning techniques attempt to predict more fine-grained local properties of proteins, such as backbone dihedral angles in unassigned regions. Both SVMs and neural networks have been applied to this problem.
More recently, real-value torsion angles can be accurately predicted by
SPINE-X and successfully employed for ab initio structure prediction.
Other improvements
It
is reported that in addition to the protein sequence, secondary
structure formation depends on other factors. For example, it is
reported that secondary structure tendencies depend also on local
environment, solvent accessibility of residues, protein structural class, and even the organism from which the proteins are obtained.
Based on such observations, some studies have shown that secondary
structure prediction can be improved by addition of information about
protein structural class, residue accessible surface area and also contact number information.
Tertiary structure
The
practical role of protein structure prediction is now more important
than ever. Massive amounts of protein sequence data are produced by
modern large-scale DNA sequencing efforts such as the Human Genome Project. Despite community-wide efforts in structural genomics, the output of experimentally determined protein structures—typically by time-consuming and relatively expensive X-ray crystallography or NMR spectroscopy—is lagging far behind the output of protein sequences.
The protein structure prediction remains an extremely difficult
and unresolved undertaking. The two main problems are calculation of protein free energy and finding the global minimum of this energy. A protein structure prediction method must explore the space of possible protein structures which is astronomically large. These problems can be partially bypassed in "comparative" or homology modeling and fold recognition
methods, in which the search space is pruned by the assumption that the
protein in question adopts a structure that is close to the
experimentally determined structure of another homologous protein. On
the other hand, the de novo or ab initio protein structure prediction
methods must explicitly resolve these problems. The progress and
challenges in protein structure prediction has been reviewed in Zhang
2008.
Ab initio protein modelling
Energy- and fragment-based methods
Ab initio- or de novo-
protein modelling methods seek to build three-dimensional protein
models "from scratch", i.e., based on physical principles rather than
(directly) on previously solved structures. There are many possible
procedures that either attempt to mimic protein folding or apply some stochastic method to search possible solutions (i.e., global optimization
of a suitable energy function). These procedures tend to require vast
computational resources, and have thus only been carried out for tiny
proteins. To predict protein structure de novo for larger
proteins will require better algorithms and larger computational
resources like those afforded by either powerful supercomputers (such as
Blue Gene or MDGRAPE-3) or distributed computing (such as Folding@home, the Human Proteome Folding Project and Rosetta@Home).
Although these computational barriers are vast, the potential benefits
of structural genomics (by predicted or experimental methods) make ab initio structure prediction an active research field.
As of 2009, a 50-residue protein could be simulated atom-by-atom on a supercomputer for 1 millisecond.
As of 2012, comparable stable-state sampling could be done on a
standard desktop with a new graphics card and more sophisticated
algorithms. A much larger simulation timescales can be achieved using coarse-grained modeling.
Evolutionary covariation to predict 3D contacts
As sequencing became more commonplace in the 1990s several groups used protein sequence alignments to predict correlated mutations
and it was hoped that these coevolved residues could be used to predict
tertiary structure (using the analogy to distance constraints from
experimental procedures such as NMR).
The assumption is when single residue mutations are slightly
deleterious, compensatory mutations may occur to restabilize
residue-residue interactions.
This early work used what are known as local methods to calculate
correlated mutations from protein sequences, but suffered from indirect
false correlations which result from treating each pair of residues as
independent of all other pairs.
In 2011, a different, and this time global statistical
approach, demonstrated that predicted coevolved residues were sufficient
to predict the 3D fold of a protein, providing there are enough
sequences available (>1,000 homologous sequences are needed). The method, EVfold,
uses no homology modeling, threading or 3D structure fragments and can
be run on a standard personal computer even for proteins with hundreds
of residues. The accuracy of the contacts predicted using this and
related approaches has now been demonstrated on many known structures
and contact maps, including the prediction of experimentally unsolved transmembrane proteins.
Comparative protein modeling
Comparative
protein modelling uses previously solved structures as starting points,
or templates. This is effective because it appears that although the
number of actual proteins is vast, there is a limited set of tertiary structural motifs
to which most proteins belong. It has been suggested that there are
only around 2,000 distinct protein folds in nature, though there are
many millions of different proteins.
These methods may also be split into two groups:
Homology modeling is based on the reasonable assumption that two homologous
proteins will share very similar structures. Because a protein's fold
is more evolutionarily conserved than its amino acid sequence, a target
sequence can be modeled with reasonable accuracy on a very distantly
related template, provided that the relationship between target and
template can be discerned through sequence alignment.
It has been suggested that the primary bottleneck in comparative
modelling arises from difficulties in alignment rather than from errors
in structure prediction given a known-good alignment. Unsurprisingly, homology modelling is most accurate when the target and template have similar sequences.
Protein threading:
scans the amino acid sequence of an unknown structure against a
database of solved structures. In each case, a scoring function is used
to assess the compatibility of the sequence to the structure, thus
yielding possible three-dimensional models. This type of method is also
known as 3D-1D fold recognition due to its compatibility analysis
between three-dimensional structures and linear protein sequences. This
method has also given rise to methods performing an inverse folding search
by evaluating the compatibility of a given structure with a large
database of sequences, thus predicting which sequences have the
potential to produce a given fold.
Side-chain geometry prediction
Accurate packing of the amino acid side chains
represents a separate problem in protein structure prediction. Methods
that specifically address the problem of predicting side-chain geometry
include dead-end elimination and the self-consistent mean field
methods. The side chain conformations with low energy are usually
determined on the rigid polypeptide backbone and using a set of discrete
side chain conformations known as "rotamers." The methods attempt to identify the set of rotamers that minimize the model's overall energy.
These methods use rotamer libraries, which are collections of
favorable conformations for each residue type in proteins. Rotamer
libraries may contain information about the conformation, its frequency,
and the standard deviations about mean dihedral angles, which can be
used in sampling. Rotamer libraries are derived from structural bioinformatics
or other statistical analysis of side-chain conformations in known
experimental structures of proteins, such as by clustering the observed
conformations for tetrahedral carbons near the staggered (60°, 180°,
-60°) values.
Rotamer libraries can be backbone-independent,
secondary-structure-dependent, or backbone-dependent.
Backbone-independent rotamer libraries make no reference to backbone
conformation, and are calculated from all available side chains of a
certain type (for instance, the first example of a rotamer library, done
by Ponder and Richards at Yale in 1987). Secondary-structure-dependent libraries present different dihedral angles and/or rotamer frequencies for -helix, -sheet, or coil secondary structures.
Backbone-dependent rotamer libraries present conformations and/or
frequencies dependent on the local backbone conformation as defined by
the backbone dihedral angles and , regardless of secondary structure.
The modern versions of these libraries as used in most software
are presented as multidimensional distributions of probability or
frequency, where the peaks correspond to the dihedral-angle
conformations considered as individual rotamers in the lists. Some
versions are based on very carefully curated data and are used primarily
for structure validation,
while others emphasize relative frequencies in much larger data sets
and are the form used primarily for structure prediction, such as the
Dunbrack rotamer libraries.
Side-chain packing methods are most useful for analyzing the protein's hydrophobic
core, where side chains are more closely packed; they have more
difficulty addressing the looser constraints and higher flexibility of
surface residues, which often occupy multiple rotamer conformations
rather than just one.
Prediction of structural classes
Statistical methods have been developed for predicting structural classes of proteins based on their amino acid composition, pseudo amino acid composition and functional domain composition.
Quaternary structure
In the case of complexes of two or more proteins, where the structures of the proteins are known or can be predicted with high accuracy, protein–protein docking
methods can be used to predict the structure of the complex.
Information of the effect of mutations at specific sites on the affinity
of the complex helps to understand the complex structure and to guide
docking methods.
Software
A great number of software tools for protein structure prediction exist. Approaches include homology modeling, protein threading, ab initio methods, secondary structure prediction, and transmembrane helix and signal peptide prediction. Some recent successful methods based on the CASP experiments include I-TASSER and HHpred. For complete list see main article.
Evaluation of automatic structure prediction servers
CASP,
which stands for Critical Assessment of Techniques for Protein Structure
Prediction, is a community-wide experiment for protein structure
prediction taking place every two years since 1994. CASP provides with
an opportunity to assess the quality of available human, non-automated
methodology (human category) and automatic servers for protein structure
prediction (server category, introduced in the CASP7).
The CAMEO3D
Continuous Automated Model EvaluatiOn Server evaluates automated
protein structure prediction servers on a weekly basis using blind
predictions for newly release protein structures. CAMEO publishes the
results on its website.