Radiation protection, also known as radiological protection, is defined by the International Atomic Energy Agency (IAEA) as "The protection of people from harmful effects of exposure to ionizing radiation,
and the means for achieving this". The IAEA also states "The accepted
understanding of the term radiation protection is restricted to
protection of people. Suggestions to extend the definition to include
the protection of non-human species or the protection of the environment
are controversial". Exposure can be from a radiation source external to the human body or due to the bodily intake of a radioactive material.
Ionizing radiation is widely used in industry and medicine, and can present a significant health hazard by causing microscopic damage to living tissue. This can result in skin burns and radiation sickness at high exposures, known as "tissue" or "deterministic" effects (conventionally indicated by the gray), and statistically elevated risks of cancer at low exposures, known as "stochastic effects" (conventionally measured by the sievert).
Fundamental to radiation protection is the reduction of expected dose and the measurement of dose uptake. For radiation protection and dosimetry assessment the International Committee on Radiation Protection (ICRP) and International Commission on Radiation Units and Measurements (ICRU) publish recommendations and data which is used to calculate the biological effects on the human body of certain levels of radiation, and thereby advise acceptable dose uptake limits. Supporting these are preventive dose reduction techniques such as radiation shielding, exposure planning and avoidance of ingestion of radioactive substances. Radiation protection instruments are used to indicate radiation hazards, and personal dosimeters and bioassay techniques are used to measure personal dose uptake.
Ionizing radiation is widely used in industry and medicine, and can present a significant health hazard by causing microscopic damage to living tissue. This can result in skin burns and radiation sickness at high exposures, known as "tissue" or "deterministic" effects (conventionally indicated by the gray), and statistically elevated risks of cancer at low exposures, known as "stochastic effects" (conventionally measured by the sievert).
Fundamental to radiation protection is the reduction of expected dose and the measurement of dose uptake. For radiation protection and dosimetry assessment the International Committee on Radiation Protection (ICRP) and International Commission on Radiation Units and Measurements (ICRU) publish recommendations and data which is used to calculate the biological effects on the human body of certain levels of radiation, and thereby advise acceptable dose uptake limits. Supporting these are preventive dose reduction techniques such as radiation shielding, exposure planning and avoidance of ingestion of radioactive substances. Radiation protection instruments are used to indicate radiation hazards, and personal dosimeters and bioassay techniques are used to measure personal dose uptake.
Principles
International policy relationships in radiological protection
External dose quantities used in radiation protection and dosimetry - based on ICRU report 57
Graphic showing relationships between radioactivity and detected ionizing radiation
The ICRP recommends, develops and maintains the International System
of Radiological Protection, based on evaluation of the large body of
scientific studies available to equate risk to received dose levels. The
system's health objectives are "to manage and control exposures to
ionising radiation so that deterministic effects are prevented, and the
risks of stochastic effects are reduced to the extent reasonably
achievable".
The ICRP's recommendations flow down to national and regional
regulators, which have the opportunity to incorporate them into their
own law; this process is shown in the accompanying block diagram. In
most countries a national regulatory authority works towards ensuring a
secure radiation environment in society by setting dose limitation
requirements that are generally based on the recommendations of the
ICRP.
Exposure situations
The ICRP recognises planned, emergency, and existing exposure situations, as described below:
- Planned exposure – defined as "...where radiological protection can be planned in advance, before exposures occur, and where the magnitude and extent of the exposures can be reasonably predicted." These are such as in occupational exposure situations, where it is necessary for personnel to work in a known radiation environment.
- Emergency exposure – defined as "...unexpected situations that may require urgent protective actions". This would be such as an emergency nuclear event.
- Existing exposure – defined as "...being those that already exist when a decision on control has to be taken". These can be such as from naturally occurring radioactive materials which exist in the environment.
Regulation of dose uptake
The ICRP uses the following overall principles for all controllable exposure situations.
- Justification: No unnecessary use of radiation is permitted, which means that the advantages must outweigh the disadvantages.
- Limitation: Each individual must be protected against risks that are too great, through the application of individual radiation dose limits.
- Optimization: This process is intended for application to those situations that have been deemed to be justified. It means "the likelihood of incurring exposures, the number of people exposed, and the magnitude of their individual doses" should all be kept as Low As Reasonably Achievable (known as ALARA or ALARP). It takes into account economic and societal factors.
Factors in external dose uptake
There are three factors that control the amount, or dose, of
radiation received from a source. Radiation exposure can be managed by a
combination of these factors:
- Time: Reducing the time of an exposure reduces the effective dose proportionally. An example of reducing radiation doses by reducing the time of exposures might be improving operator training to reduce the time they take to handle a radioactive source.
- Distance: Increasing distance reduces dose due to the inverse square law. Distance can be as simple as handling a source with forceps rather than fingers.
- Shielding: Sources of radiation can be shielded with solid or liquid material, which absorbs the energy of the radiation. The term 'biological shield' is used for absorbing material placed around a nuclear reactor, or other source of radiation, to reduce the radiation to a level safe for humans.
Internal dose uptake
Large scale glovebox in the nuclear industry used to contain airborne radioactive particles.
Internal dose, due to the inhalation or ingestion of radioactive
substances, can result in stochastic or deterministic effects, depending
on the amount of radioactive material ingested and other biokinetic factors.
The risk from a low level internal source is represented by the dose quantity committed dose, which has the same risk as the same amount of external effective dose.
The intake of radioactive material can occur through four pathways:
- Inhalation of airborne contaminants such as radon gas and radioactive particles
- Ingestion of radioactive contamination in food or liquids
- Absorption of vapours such as tritium oxide through the skin
- Injection of medical radioisotopes such as technetium-99m
The occupational hazards from airborne radioactive particles in
nuclear and radio-chemical applications are greatly reduced by the
extensive use of gloveboxes to contain such material. To protect against breathing in radioactive particles in ambient air, respirators with particulate filters are worn.
To monitor the concentration of radioactive particles in ambient air, radioactive particulate monitoring instruments measure the concentration or presence of airborne materials.
For ingested radioactive materials in food and drink, specialist
laboratory radiometric assay methods are used to measure the
concentration of such materials.
Recommended limits on dose uptake
USA Dept of Energy 2010 dose chart in sieverts for a variety of situations and applications.
Various doses of radiation in sieverts, ranging from trivial to lethal.
The ICRP recommends a number of limits for dose uptake in table 8 of
ICRP report 103. These limits are "situational", for planned, emergency
and existing situations. Within these situations, limits are given for
certain exposed groups:
- Planned exposure – limits given for occupational, medical and public exposure. The occupational exposure limit of effective dose is 20 mSv per year, averaged over defined periods of 5 years, with no single year exceeding 50 mSv. The public exposure limit is 1 mSv in a year.
- Emergency exposure – limits given for occupational and public exposure
- Existing exposure – reference levels for all persons exposed
Further detail of some of the limits can be found on the ICRPedia page.
The public information dose chart of the USA Department of
Energy, shown here on the right, applies to USA regulation, which is
based on ICRP recommendations. Note that examples in lines 1 to 4 have a
scale of dose rate (radiation per unit time), whilst 5 and 6 have a
scale of total accumulated dose.
ALARP & ALARA
ALARP is an acronym for an important principle in exposure to
radiation and other occupational health risks and in the UK stands for "As Low As Reasonably Practicable". The aim is to minimize the risk of radioactive exposure
or other hazard while keeping in mind that some exposure may be
acceptable in order to further the task at hand. The equivalent term ALARA, "As Low As Reasonably Achievable", is more commonly used outside the UK.
This compromise is well illustrated in radiology. The application of radiation
can aid the patient by providing doctors and other health care
professionals with a medical diagnosis, but the exposure of the patient
should be reasonably low enough to keep the statistical probability of cancers or sarcomas
(stochastic effects) below an acceptable level, and to eliminate
deterministic effects (e.g. skin reddening or cataracts). An acceptable
level of incidence of stochastic effects is considered to be equal for a
worker to the risk in other radiation work generally considered to be
safe.
This policy is based on the principle that any amount of
radiation exposure, no matter how small, can increase the chance of
negative biological effects such as cancer.
It is also based on the principle that the probability of the
occurrence of negative effects of radiation exposure increases with
cumulative lifetime dose. These ideas are combined to form the linear no-threshold model
which says that there is not a threshold at which there is an increase
in the rate of occurrence of stochastic effects with increasing dose. At
the same time, radiology and other practices that involve use of
ionizing radiation bring benefits, so reducing radiation exposure can
reduce the efficacy of a medical practice. The economic cost, for
example of adding a barrier against radiation, must also be considered
when applying the ALARP principle. Computed Tomography, better known as C.T. Scans or CAT Scans have made an enormous contribution to medicine, however not without some risk. They use ionizing radiation which can cause cancer, especially in children. When caregivers follow proper indications for their use and child safe techniques rather than adult techniques, downstream cancer can be prevented.
Personal radiation dosimeters
The radiation dosimeter is an important personal dose measuring
instrument. It is worn by the person being monitored and is used to
estimate the external radiation dose deposited in the individual wearing
the device. They are used for Gamma, X-ray, beta and other strongly
penetrating radiation, but not for weakly penetrating radiation such as
alpha particles. Traditionally film badges were used for long term
monitoring, and quartz fibre dosimeters for short term monitoring.
However, these are mostly superseded by such as thermoluminescent
dosimetry (TLD) badges and electronic dosimeters. Electronic dosimeters
can give an alarm warning if a preset dose threshold has been reached,
enabling safer working in potentially higher radiation levels, where the
received dose must be continually monitored.
Workers exposed to radiation, such as radiographers, nuclear power plant workers, doctors using radiotherapy, those in laboratories using radionuclides, and HAZMAT
teams are required to wear dosimeters so a record of occupational
exposure can be made. Such devices are generally termed "legal
dosimeters" if they have been approved for use in recording personnel
dose for regulatory purposes.
Dosimeters can be worn to obtain a whole body dose and there are
also specialist types that can be worn on the fingers or clipped to
headgear, to measure the localised body irradiation for specific
activities.
Common types of wearable dosimeters for ionizing radiation include:
- Film badge dosimeter
- Quartz fiber dosimeter
- Electronic personal dosimeter
- Thermoluminescent dosimeter
Radiation shielding
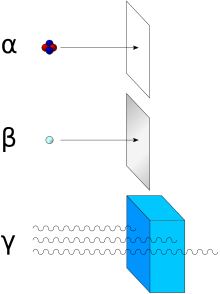
Diagram showing various forms of ionizing radiation, and the sort of material that is used to stop or reduce that type.
The
total absorption coefficient of lead (atomic number 82) for gamma rays,
plotted versus gamma energy, and the contributions by the three
effects. Here, the photoelectric effect dominates at low energy. Above 5
MeV, pair production starts to dominate.
A lead castle built to shield a radioactive sample in a lab
Almost any material can act as a shield from gamma or x-rays if used in sufficient amounts. Different types of ionizing radiation interact in different ways with shielding material. The effectiveness of shielding is dependent on the Stopping power of radiation particles,
which varies with the type and energy of radiation and the shielding
material used. Different shielding techniques are therefore used
dependent on the application and the type and energy of the radiation.
Shielding reduces the intensity of radiation depending on the
thickness. This is an exponential relationship with gradually
diminishing effect as equal slices of shielding material are added. A
quantity known as the halving-thicknesses is used to calculate this. For example, a practical shield in a fallout shelter with ten halving-thicknesses of packed dirt, which is roughly 115 cm (3 ft 9 in) reduces gamma rays to 1/1024 of their original intensity (i.e. 1/210).
The effectiveness of a shielding material in general increases with its atomic number, called Z, except for neutron shielding which is more readily shielded by the likes of neutron absorbers and moderators such as compounds of boron e.g. boric acid, cadmium, carbon and hydrogen respectively.
Graded-Z shielding is a laminate of several materials with different Z values (atomic numbers) designed to protect against ionizing radiation. Compared to single-material shielding, the same mass of graded-Z shielding has been shown to reduce electron penetration over 60%. It is commonly used in satellite-based particle detectors, offering several benefits:
- Protection from radiation damage
- Reduction of background noise for detectors
- Lower mass compared to single-material shielding
Designs vary, but typically involve a gradient from high-Z (usually tantalum) through successively lower-Z elements such as tin, steel, and copper, usually ending with aluminium. Sometimes even lighter materials such as polypropylene or boron carbide are used.
In a typical graded-Z shield, the high-Z layer effectively scatters protons and electrons. It also absorbs gamma rays, which produces X-ray fluorescence.
Each subsequent layer absorbs the X-ray fluorescence of the previous
material, eventually reducing the energy to a suitable level. Each
decrease in energy produces bremsstrahlung and Auger electrons,
which are below the detector's energy threshold. Some designs also
include an outer layer of aluminium, which may simply be the skin of the
satellite.
The effectiveness of a material as a biological shield is related to its
cross-section for scattering and absorption,
and to a first approximation is proportional to the total mass of
material per unit area interposed along the line of sight between the
radiation source and the region to be protected. Hence, shielding
strength or "thickness" is conventionally measured in units of g/cm2. The radiation that manages to get through falls exponentially with the thickness of the shield. In x-ray facilities, walls surrounding the room with the x-ray generator may contain lead sheets, or the plaster may contain barium sulfate. Operators view the target through a leaded glass screen, or if they must remain in the same room as the target, wear lead aprons.
Particle radiation
Particle radiation consists of a stream of charged or neutral particles, both charged ions and subatomic elementary particles. This includes solar wind, cosmic radiation, and neutron flux in nuclear reactors.
- Alpha particles (helium nuclei) are the least penetrating. Even very energetic alpha particles can be stopped by a single sheet of paper.
- Beta particles (electrons) are more penetrating, but still can be absorbed by a few millimeters of aluminum. However, in cases where high energy beta particles are emitted shielding must be accomplished with low atomic weight materials, e.g. plastic, wood, water, or acrylic glass (Plexiglas, Lucite). This is to reduce generation of Bremsstrahlung X-rays. In the case of beta+ radiation (positrons), the gamma radiation from the electron-positron annihilation reaction poses additional concern.
- Neutron radiation is not as readily absorbed as charged particle radiation, which makes this type highly penetrating. Neutrons are absorbed by nuclei of atoms in a nuclear reaction. This most often creates a secondary radiation hazard, as the absorbing nuclei transmute to the next-heavier isotope, many of which are unstable.
- Cosmic radiation is not a common concern, as the Earth's atmosphere absorbs it and the magnetosphere acts as a shield, but it poses a problem for satellites and astronauts. Frequent fliers are also at a slight risk. Cosmic radiation is extremely high energy, and is very penetrating.
Electromagnetic radiation
Electromagnetic radiation consists of emissions of electromagnetic waves, the properties of which depend on the wavelength.
- X-ray and gamma radiation are best absorbed by atoms with heavy nuclei; the heavier the nucleus, the better the absorption. In some special applications, depleted uranium or thorium are used, but lead is much more common; several centimeters are often required. Barium sulfate is used in some applications too. However, when cost is important, almost any material can be used, but it must be far thicker. Most nuclear reactors use thick concrete shields to create a bioshield with a thin water cooled layer of lead on the inside to protect the porous concrete from the coolant inside. The concrete is also made with heavy aggregates, such as Baryte or MagnaDense (Magnetite), to aid in the shielding properties of the concrete. Gamma rays are better absorbed by materials with high atomic numbers and high density, although neither effect is important compared to the total mass per area in the path of the gamma ray.
- Ultraviolet (UV) radiation is ionizing in its shortest wavelengths but it is not penetrating, so it can be shielded by thin opaque layers such as sunscreen, clothing, and protective eyewear. Protection from UV is simpler than for the other forms of radiation above, so it is often considered separately.
In some cases, improper shielding can actually make the situation
worse, when the radiation interacts with the shielding material and
creates secondary radiation that absorbs in the organisms more readily.
For example, although high atomic number materials are very effective in
shielding photons, using them to shield beta particles may cause higher radiation exposure due to the production of bremsstrahlung x-rays, and hence low atomic number materials are recommended. Also, using material with a high neutron activation cross section
to shield neutrons will result in the shielding material itself
becoming radioactive and hence more dangerous than if it were not
present.
Personal Protective Equipment (PPE) - Radiation
Personal Protection Equipment
(PPE) includes all clothing and accessories which can be worn to
prevent severe illness and injury as a result of exposure to radioactive
material. Because radiation can affect humans through internal and
external contamination, various protection strategies have been
developed to protect humans from the harmful effects of radiation
exposure from a spectrum of sources. A few of these strategies developed to shield from internal, external, and high energy radiation are outlined below.
Internal Contamination Protective Equipment
Internal
contamination protection equipment protects against the inhalation and
ingestion of radioactive material. Internal deposition of radioactive
material result in direct exposure of radiation to organs and tissues
inside the body. The respiratory protective equipment described below
are designed to minimize the possibility of such material being inhaled
or ingested as emergency workers are exposed to potentially radioactive
environments.
Reusable Air Purifying Respirators (APR)
- Elastic face piece worn over the mouth and nose
- Contains filters, cartridges, and canisters to provide increased protection and better filtration
- Battery powered blower forces contamination through air purifying filters
- Purified air delivered under positive pressure to face piece
Supplied-Air Respirator (SAR)
- Compressed air delivered from a stationary source to the face piece
Auxiliary Escape Respirator
- Protects wearer from breathing harmful gases, vapors, fumes, and dust
- Can be designed as an air-purifying escape respirator (APER) or a self-contained breathing apparatus (SCBA) type respirator
- SCBA type escape respirators have an attached source of breathing air and a hood that provides a barrier against contaminated outside air
- Provides very pure, dry compressed air to full facepiece mask via a hose
- Air is exhaled to environment
- By law, must be worn whenever entering environments immediately dangerous to life and health (IDLH) or when information is inadequate to rule out IDLH atmosphere
External Contamination Protective Equipment
External
contamination protection equipment provides a barrier to shield
radioactive material from being deposited externally on the body or
clothes. The dermal protective equipment described below acts as a
barrier to block radioactive material from physically touching the skin,
but does not protect against externally penetrating high energy
radiation.
Chemical- Resistant Inner Suit
- Porous overall suit - Dermal protection from aerosols, dry particles, and non hazardous liquids.
- Non-porous overall suit to provide dermal protection from:
- Dry powders and solids
- Blood-borne pathogens and bio-hazards
- Chemical splashes and inorganic acid/base aerosols
- Mild Liquid Chemical Splashes from toxics and corrosices
- Toxic industrial chemicals and materials
Level C Equivalent: Bunker Gear
- Fire fighter protective clothing
- Flame/water resistant
- Helmet, gloves, foot gear, and hood
Level B Equivalent - Non-gas-tight Encapsulating Suit
- Designed for environments which are immediately health risks but contain no substances which can be absorbed by skin
Level A Equivalent - Totally Encapsulating Chemical - and Vapor Protective Suit
- Designed for environments which are immediate health risks and contain substances which can be absorbed by skin
External penetrating radiation
Many solutions to low energy radiation exposure like X-rays already exist. Lead aprons,
for example, can protect patients and clinicians from the potentially
harmful radiation effects of day to day medical examinations. It is
quite feasible to protect large surface areas of the body from radiation
in the lower energy spectrum because very little shielding material is
required to provide the necessary protection.
Personal shielding against more energetic radiation such as gamma radiation
is very difficult to achieve as the large mass of shielding material
required to properly protect the entire body would make functional
movement nearly impossible. For this, partial body shielding of
radio-sensitive internal organs is the most viable protection strategy.
The immediate danger of intense exposure to high energy gamma radiation is Acute Radiation Syndrome (ARS), a result of irreversible bone marrow damage. The concept of selective shielding is based in the regenerative potential of the hematopoietic stem cells
found in bone marrow. The regenerative quality of stem cells make it
only necessary to protect enough bone marrow to repopulate the body with
unaffected stem cells after the exposure: a similar concept which is
applied in hematopoietic stem cell transplantation (HSCT)
which is a common treatment for patients suffering from leukemia. This
scientific advancement allows for the development of a new class of
relatively light weight protective equipment which shields high
concentrations of bone marrow to defer the hematopoietic sub-syndrome of
Acute Radiation Syndrome to much higher dosages.
One technique is to apply selective shielding to protect the high
concentration of bone marrow stored in the hips and other
radio-sensitive organs in the abdominal area. This allows first
responders a safe way to perform necessary missions in radioactive
environments. The Organisation for Economic Co-operation and Development (OECD) and the Nuclear Energy Agency (NEA) have published a brief section outlining the benefits of partial body shielding in the 2015 report: Occupational Radiation Protection in Severe Accident Management.
Radiation protection instruments
Practical
radiation measurement using calibrated radiation protection instruments
is essential in evaluating the effectiveness of protection measures,
and in assessing the radiation dose likely to be received by
individuals. The measuring instruments for radiation protection are both
"installed" (in a fixed position) and portable (hand-held or
transportable).
Installed instruments
Installed
instruments are fixed in positions which are known to be important in
assessing the general radiation hazard in an area. Examples are
installed "area" radiation monitors, Gamma interlock monitors, personnel
exit monitors, and airborne particulate monitors.
The area radiation monitor will measure the ambient radiation,
usually X-Ray, Gamma or neutrons; these are radiations which can have
significant radiation levels over a range in excess of tens of metres
from their source, and thereby cover a wide area.
Gamma radiation "interlock monitors" are used in applications to
prevent inadvertent exposure of workers to an excess dose by preventing
personnel access to an area when a high radiation level is present.
These interlock the process access directly.
Airborne contamination monitors
measure the concentration of radioactive particles in the ambient air
to guard against radioactive particles being ingested, or deposited in
the lungs of personnel. These instruments will normally give a local
alarm, but are often connected to an integrated safety system so that
areas of plant can be evacuated and personnel are prevented from
entering an air of high airborne contamination.
Personnel exit monitors (PEM) are used to monitor workers
who are exiting a "contamination controlled" or potentially contaminated
area. These can be in the form of hand monitors, clothing frisk probes,
or whole body monitors. These monitor the surface of the workers body
and clothing to check if any radioactive contamination has been deposited. These generally measure alpha or beta or gamma, or combinations of these.
The UK National Physical Laboratory
publishes a good practice guide through its Ionising Radiation
Metrology Forum concerning the provision of such equipment and the
methodology of calculating the alarm levels to be used.
Portable instruments
Hand-held
ion chamber survey meter in use for surface dose rate on one of three
radioisotope thermoelectric generators (RTGs) for the Cassini
spacecraft.
Portable instruments are hand-held or transportable.
The hand-held instrument is generally used as a survey meter
to check an object or person in detail, or assess an area where no
installed instrumentation exists. They can also be used for personnel
exit monitoring or personnel contamination checks in the field. These
generally measure alpha, beta or gamma, or combinations of these.
Transportable instruments are generally instruments that would
have been permanently installed, but are temporarily placed in an area
to provide continuous monitoring where it is likely there will be a
hazard. Such instruments are often installed on trolleys to allow easy
deployment, and are associated with temporary operational situations.
In the United Kingdom the HSE has issued a user guidance note on selecting the correct radiation measurement instrument for the application concerned. This covers all radiation instrument technologies, and is a useful comparative guide.
Instrument types
A number of commonly used detection instrument types are listed below, and are used for both fixed and survey monitoring.
- ionization chambers
- proportional counters
- Geiger counters
- Semiconductor detectors
- Scintillation detectors
- Airborne particulate radioactivity monitoring
The links should be followed for a fuller description of each.
The following table shows the main radiation related quantities and units.
| Quantity | Unit | Symbol | Derivation | Year | SI equivalence |
|---|---|---|---|---|---|
| Activity (A) | curie | Ci | 3.7 × 1010 s−1 | 1953 | 3.7×1010 Bq |
| becquerel | Bq | s−1 | 1974 | SI unit | |
| rutherford | Rd | 106 s−1 | 1946 | 1,000,000 Bq | |
| Exposure (X) | röntgen | R | esu / 0.001293 g of air | 1928 | 2.58 × 10−4 C/kg |
| Absorbed dose (D) | erg |
|
erg⋅g−1 | 1950 | 1.0 × 10−4 Gy |
| rad | rad | 100 erg⋅g−1 | 1953 | 0.010 Gy | |
| gray | Gy | J⋅kg−1 | 1974 | SI unit | |
| Dose equivalent (H) | röntgen equivalent man | rem | 100 erg⋅g−1 | 1971 | 0.010 Sv |
| sievert | Sv | J⋅kg−1 × WR | 1977 | SI unit |
Spacecraft radiation challenges
Spacecraft,
both manned and unmanned, must cope with the high radiation environment
of outerspace. Radiation emitted by the Sun and other galactic sources, and trapped in radiation "belts"
is more dangerous and hundreds of times more intense than radiation
sources such as medical X-rays or normal cosmic radiation usually
experienced on Earth.
When the intensely ionizing particles found in space strike human
tissue, it can result in cell damage and may eventually lead to cancer.
The usual method for radiation protection is material shielding
by spacecraft and equipment structures (usually aluminium), possibly
augmented by polyethylene in human spaceflight where the main concern is
high energy protons and cosmic ray ions. On unmanned spacecraft in high
electron dose environments such as Jupiter missions, or medium Earth
orbit (MEO), additional shielding with materials of a high atomic number
can be effective. On long duration manned missions, advantage can be
taken of the good shielding characteristics of liquid hydrogen fuel and
water.
The NASA Space Radiation Laboratory
makes use of a particle accelerator that produces beams of protons or
heavy ions. These ions are typical of those accelerated in cosmic
sources and by the Sun. The beams of ions move through a 100-meter
(328-foot) transport tunnel to the 37-square-meter (400-square-foot)
shielded target hall. There, they hit the target, which may be a
biological sample or shielding material. In a 2002 NASA study, it was determined that materials that have high hydrogen contents, such as polyethylene, can reduce primary and secondary radiation to a greater extent than metals, such as aluminum. The problem with this "passive shielding" method is that radiation interactions in the material generate secondary radiation.
Active Shielding, that is, using magnets, high voltages, or
artificial magnetospheres to slow down or deflect radiation, has been
considered to potentially combat radiation in a feasible way. So far,
the cost of equipment, power and weight of active shielding equipment
outweigh their benefits. For example, active radiation equipment would
need a habitable volume size to house it, and magnetic and electrostatic
configurations often are not homogenous in intensity, allowing
high-energy particles to penetrate the magnetic and electric fields from
low-intensity parts, like cusps in dipolar magnetic field of Earth. As
of 2012, NASA is undergoing research in superconducting magnetic architecture for potential active shielding applications.
Early radiation dangers
Using early Crookes tube X-Ray apparatus in 1896. One man is viewing his hand with a fluoroscope to optimise tube emissions, the other has his head close to the tube. No precautions are being taken.
Monument to the X-ray and Radium Martyrs of All Nations erected 1936 at St. Georg hospital in Hamburg, commemorating 359 early radiology workers.
The dangers of radioactivity and radiation were not immediately
recognized. The discovery of x‑rays in 1895 led to widespread
experimentation by scientists, physicians, and inventors. Many people
began recounting stories of burns, hair loss and worse in technical
journals as early as 1896. In February of that year, Professor Daniel
and Dr. Dudley of Vanderbilt University
performed an experiment involving x-raying Dudley's head that resulted
in his hair loss. A report by Dr. H.D. Hawks, a graduate of Columbia
College, of his suffering severe hand and chest burns in an x-ray
demonstration, was the first of many other reports in Electrical Review.
Many experimenters including Elihu Thomson at Thomas Edison's lab, William J. Morton, and Nikola Tesla
also reported burns. Elihu Thomson deliberately exposed a finger to an
x-ray tube over a period of time and suffered pain, swelling, and
blistering. Other effects, including ultraviolet rays and ozone were sometimes blamed for the damage. Many physicists claimed that there were no effects from x-ray exposure at all.
As early as 1902 William Herbert Rollins
wrote almost despairingly that his warnings about the dangers involved
in careless use of x-rays was not being heeded, either by industry or by
his colleagues. By this time Rollins had proved that x-rays could kill
experimental animals, could cause a pregnant guinea pig to abort, and
that they could kill a fetus.
He also stressed that "animals vary in susceptibility to the external
action of X-light" and warned that these differences be considered when
patients were treated by means of x-rays.
Before the biological effects of radiation were known, many
physicists and corporations began marketing radioactive substances as patent medicine in the form of glow-in-the-dark pigments. Examples were radium enema treatments, and radium-containing waters to be drunk as tonics. Marie Curie
protested against this sort of treatment, warning that the effects of
radiation on the human body were not well understood. Curie later died
from aplastic anaemia,
likely caused by exposure to ionizing radiation. By the 1930s, after a
number of cases of bone necrosis and death of radium treatment
enthusiasts, radium-containing medicinal products had been largely
removed from the market (radioactive quackery).