| African trypanosomiasis | |
|---|---|
| Other names | Sleeping sickness, African sleeping sickness |
 | |
| Trypanosoma forms in a blood smear | |
| Specialty | Infectious disease |
| Symptoms | Stage 1: Fevers, headaches, itchiness, joint pains Stage 2: Trouble sleeping, confusion, poor coordination |
| Usual onset | 1–3 weeks post exposure |
| Types | Trypanosoma brucei gambiense (TbG), Trypanosoma brucei rhodesiense (TbR) |
| Causes | Trypanosoma brucei spread by tsetse flies |
| Diagnostic method | Blood smear, lumbar puncture |
| Medication | Fexinidazole, pentamidine, suramin, eflornithine, nifurtimox |
| Prognosis | Fatal without treatment |
| Frequency | 977 (2018) |
| Deaths | 3,500 (2015) |
African trypanosomiasis, also known as African sleeping sickness or simply sleeping sickness, is an insect-borne parasitic infection of humans and other animals. It is caused by the species Trypanosoma brucei. Humans are infected by two types, Trypanosoma brucei gambiense (TbG) and Trypanosoma brucei rhodesiense (TbR). TbG causes over 98% of reported cases. Both are usually transmitted by the bite of an infected tsetse fly and are most common in rural areas.
Initially, the first stage of the disease is characterized by fevers, headaches, itchiness, and joint pains, beginning one to three weeks after the bite. Weeks to months later, the second stage begins with confusion, poor coordination, numbness, and trouble sleeping. Diagnosis is by finding the parasite in a blood smear or in the fluid of a lymph node. A lumbar puncture is often needed to tell the difference between first- and second-stage disease. If the disease is not treated quickly it can lead to death.
Prevention of severe disease involves screening the at-risk population with blood tests for TbG. Treatment is easier when the disease is detected early and before neurological symptoms occur. Treatment of the first stage has been with the medications pentamidine or suramin. Treatment of the second stage has involved eflornithine or a combination of nifurtimox and eflornithine for TbG. Fexinidazole is a more recent treatment that can be taken by mouth, for either stage of TbG. While melarsoprol works for both types, it is typically only used for TbR, due to serious side effects. Without treatment, sleeping sickness typically results in death.
The disease occurs regularly in some regions of sub-Saharan Africa with the population at risk being about 70 million in 36 countries. An estimated 11,000 people are currently infected with 2,800 new infections in 2015. In 2018 there were 977 new cases. In 2015 it caused around 3,500 deaths, down from 34,000 in 1990. More than 80% of these cases are in the Democratic Republic of the Congo. Three major outbreaks have occurred in recent history: one from 1896 to 1906 primarily in Uganda and the Congo Basin, and two in 1920 and 1970, in several African countries. It is classified as a neglected tropical disease. Other animals, such as cows, may carry the disease and become infected in which case it is known as Nagana or animal trypanosomiasis.
Signs and symptoms
African trypanosomiasis symptoms occur in two stages: the hemolymphatic stage and the neurological stage (the latter being characterised by parasitic invasion of the central nervous system). Neurological symptoms occur in addition to the initial features, however, and the two stages may be difficult to distinguish based on clinical features alone.
The disease has been reported to present with atypical symptoms in infected individuals who originate from non-endemic areas (e.g. travelers). The reasons for this are unclear and may be genetic. The low number of such cases may also have skewed findings. In such persons, the infection is said to present mainly as fever with gastrointestinal symptoms (e.g. diarrhoea and jaundice) with lymphadenopathy developing only rarely.
Trypanosomal chancre
Systemic disease is sometimes presaged by a trypanosomal chancre developing at the site of the infectious fly bite within 2 days of infection. The chancre is most commonly observed in T. b. rhodesiense infection, and only rarely in T. b. gambiense (however, in T. b. gambiense infection, chancres are more common in persons from non-endemic areas).
Hemolymphatic phase
Incubation period is 1–3 weeks for T. b. rhodesiense, and longer (but less precisely characterised) in T. b. gambiense infection. The first/initial stage, known as the hemolymphatic phase, is characterized by non-specific, generalised symptoms like: fever (intermittent), headaches (severe), joint pains, itching, weakness, malaise, fatigue, weight loss, lymphadenopathy, and hepatosplenomegaly.
Diagnosis may be delayed due to the vagueness of initial symptoms. The disease may also be mistaken for malaria (which may in fact occur as a co-infection).
Intermittent fever
Fever is intermittent, with attacks lasting from a day to a week, separated by intervals of a few days to a month or longer. Episodes of fever become less frequent over the course of the disease.
Lymphadenopathy
Invasion of the circulatory and lymphatic systems by the parasite is associated with severe swelling of lymph nodes, often to tremendous sizes. Posterior cervical lymph nodes are most commonly affected, however, axillary, inguinal, and epitrochlear lymph node involvement may also occur. Winterbottom's sign, the tell-tale swollen lymph nodes along the back of the neck, may appear. Winterbottom's sign is common in T. b. gambiense infection.
Other features
Those affected may additionally present with: skin rash, haemolytic anaemia, hepatomegaly and abnormal liver function, splenomegaly, endocrine disturbance, cardiac involvement (e.g. pericarditis, and congestive heart failure), and ophthalmic involvement.
Numerous spots of bleeding into the skin of the leg in a person infected with T. b. rhodesiense
Neurological phase
The second phase of the disease, the neurological phase (also called the meningoencephalic stage), begins when the parasite invades the central nervous system by passing through the blood–brain barrier. Progression to the neurological phase occurs after an estimated 21–60 days in case of T. b. rhodesiense infection, and 300–500 days in case of T. b. gambiense infection.
In actuality, the two phases overlap and are difficult to distinguish based on clinical features alone; determining the actual stage of the disease is achieved by examining the cerebrospinal fluid for the presence of the parasite.
Sleep disorders
Sleep-wake disturbances are a leading feature of neurological stage and gave the disease its common name African sleeping sickness. Infected individuals experience a disorganized and fragmented sleep-wake cycle. Those affected experience sleep inversion resulting in daytime sleep and somnolence, and nighttime periods of wakefulness and insomnia. Additionally, those affected also experience episodes of sudden sleepiness.
Neurological/neurocognitive symptoms
Neurological symptoms include: tremor, general muscle weakness, hemiparesis, paralysis of a limb, abnormal muscle tone, gait disturbance, ataxia, speech disturbances, paraesthesia, hyperaesthesia, anaesthesia, visual disturbance, abnormal reflexes, seizures, and coma. Parkinson-like movements might arise due to non-specific movement disorders and speech disorders.
Psychiatric/behavioural symptoms
Individuals may exhibit psychiatric symptoms which may sometimes dominate the clinical diagnosis and may include aggressiveness, apathy, irritability, psychotic reactions and hallucinations, anxiety, emotional lability, confusion, mania, attention deficit, and delirium.
Advanced/late disease and outcomes
Without treatment, the disease is invariably fatal, with progressive mental deterioration leading to coma, systemic organ failure, and death. An untreated infection with T. b. rhodesiense will cause death within months whereas an untreated infection with T. b. gambiense will cause death after several years. Damage caused in the neurological phase is irreversible.
Cause
Trypanosoma brucei gambiense accounts for the majority of African trypanosomiasis cases, with humans as the main reservoir needed for the transmission, while Trypanosoma brucei rhodesiense is mainly zoonotic, with the occasional human infection. African trypanosomiasis is dependent on the interaction of the parasite (trypanosome) with the tsetse flies (vector), as well as the host (human for Trypanosoma brucei gambiense, and animals for Trypanosoma brucei rhodesiense). The risk of contracting African trypanosomiasis is dependent on coming in contact with an infected tsetse fly.
Trypanosoma brucei
There are two subspecies of the parasite that are responsible for starting the disease in humans. Trypanosoma brucei gambiense causes the diseases in west and central Africa, whereas Trypanosoma brucei rhodesiense has a limited geographical range and is responsible for causing the disease in east and southern Africa. In addition, a third subspecies of the parasite known as Trypanosoma brucei brucei is responsible for affecting animals but not humans.
Humans are the main reservoir for T. b. gambiense but this species can also be found in pigs and other animals. Wild game animals and cattle are the main reservoir of T. b. rhodesiense. These parasites primarily infect individuals in sub-Saharan Africa because that is where the vector (tsetse fly) is located. The two human forms of the disease also vary greatly in intensity. T. b. gambiense causes a chronic condition that can remain in a passive phase for months or years before symptoms emerge and the infection can last about three years before death occurs.
T. b. rhodesiense is the acute form of the disease, and death can occur within months since the symptoms emerge within weeks and it is more virulent and faster developing than T. b. gambiense. Furthermore, trypanosomes are surrounded by a coat that is composed of variant surface glycoproteins (VSG). These proteins act to protect the parasite from any lytic factors that are present in human plasma. The host's immune system recognizes the glycoproteins present on the coat of the parasite leading to the production of different antibodies (IgM and IgG).
These antibodies will then act to destroy the parasites that circulate around the blood. However, from the several parasites present in the plasma, a small number of them will experience changes in their surface coats resulting in the formation of new VSGs. Thus, the antibodies produced by the immune system will no longer recognize the parasite leading to proliferation until new antibodies are created to combat the novel VSGs. Eventually, the immune system will no longer be able to fight off the parasite due to the constant changes in VSGs and infection will arise.
Vector
| Type | Trypanosoma | Distribution | Vector |
|---|---|---|---|
| Chronic | T. brucei gambiense | Western Africa | G. palpalis
G. tachinoides G. morsitans |
| Acute | T. brucei rhodesiense | Eastern Africa | G. morsitans
G. swynnertoni G. pallidipes G. fuscipes |
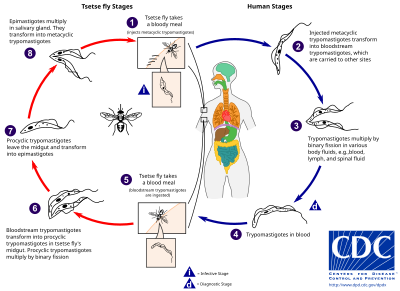
The tsetse fly (genus Glossina) is a large, brown, biting fly that serves as both a host and vector for the trypanosome parasites. While taking blood from a mammalian host, an infected tsetse fly injects metacyclic trypomastigotes into skin tissue. From the bite, parasites first enter the lymphatic system and then pass into the bloodstream. Inside the mammalian host, they transform into bloodstream trypomastigotes, and are carried to other sites throughout the body, reach other body fluids (e.g., lymph, spinal fluid), and continue to replicate by binary fission.
The entire life cycle of African trypanosomes is represented by extracellular stages. A tsetse fly becomes infected with bloodstream trypomastigotes when taking a blood meal on an infected mammalian host. In the fly's midgut, the parasites transform into procyclic trypomastigotes, multiply by binary fission, leave the midgut, and transform into epimastigotes. The epimastigotes reach the fly's salivary glands and continue multiplication by binary fission.
The entire life cycle of the fly takes about three weeks. In addition to the bite of the tsetse fly, the disease can be transmitted by:
- Mother-to-child infection: the trypanosome can sometimes cross the placenta and infect the fetus.
- Laboratories: accidental infections, for example, through the handling of blood of an infected person and organ transplantation, although this is uncommon.
- Blood transfusion
- Sexual contact
Horse-flies (Tabanidae) and stable flies (Muscidae) possibly play a role in transmission of nagana (the animal form of sleeping sickness) and the human disease form.
Pathophysiology
Tryptophol is a chemical compound produced by the trypanosomal parasite in sleeping sickness which induces sleep in humans.
Diagnosis
The gold standard for diagnosis is identification of trypanosomes in a sample by microscopic examination. Samples that can be used for diagnosis include chancre fluid, lymph node aspirates, blood, bone marrow, and, during the neurological stage, cerebrospinal fluid. Detection of trypanosome-specific antibodies can be used for diagnosis, but the sensitivity and specificity of these methods are too variable to be used alone for clinical diagnosis. Further, seroconversion occurs after the onset of clinical symptoms during a T. b. rhodesiense infection, so is of limited diagnostic use.
Trypanosomes can be detected from samples using two different preparations. A wet preparation can be used to look for the motile trypanosomes. Alternatively, a fixed (dried) smear can be stained using Giemsa's or Field's technique and examined under a microscope. Often, the parasite is in relatively low abundance in the sample, so techniques to concentrate the parasites can be used prior to microscopic examination. For blood samples, these include centrifugation followed by examination of the buffy coat; mini anion-exchange/centrifugation; and the quantitative buffy coat (QBC) technique. For other samples, such as spinal fluid, concentration techniques include centrifugation followed by examination of the sediment.
Three serological tests are also available for detection of the parasite: the micro-CATT (card agglutination test for trypanosomiasis), wb-CATT, and wb-LATEX. The first uses dried blood, while the other two use whole blood samples. A 2002 study found the wb-CATT to be the most efficient for diagnosis, while the wb-LATEX is a better exam for situations where greater sensitivity is required.
Prevention
Currently there are few medically related prevention options for African trypanosomiasis (i.e. no vaccine exists for immunity). Although the risk of infection from a tsetse fly bite is minor (estimated at less than 0.1%), the use of insect repellants, wearing long-sleeved clothing, avoiding tsetse-dense areas, implementing bush clearance methods and wild game culling are the best options to avoid infection available for local residents of affected areas.
In July 2000, a resolution was passed to form the Pan African Tsetse and Trypanosomiasis Eradication Campaign (PATTEC). The campaign works to eradicate the tsetse vector population levels and subsequently the protozoan disease, by use of insecticide-impregnated targets, fly traps, insecticide-treated cattle, ultra-low dose aerial/ground spraying (SAT) of tsetse resting sites and the sterile insect technique (SIT). The use of SIT in Zanzibar proved effective in eliminating the entire population of tsetse flies but was expensive and is relatively impractical to use in many of the endemic countries afflicted with African trypanosomiasis.
A pilot program in Senegal has reduced the tsetse fly population by as much as 99% by introducing male flies which have been sterilized by exposure to gamma rays.
Regular active surveillance, involving detection and prompt treatment of new infections, and tsetse fly control is the backbone of the strategy used to control sleeping sickness. Systematic screening of at-risk communities is the best approach, because case-by-case screening is not practical in endemic regions. Systematic screening may be in the form of mobile clinics or fixed screening centres where teams travel daily to areas of high infection rates. Such screening efforts are important because early symptoms are not evident or serious enough to warrant people with gambiense disease to seek medical attention, particularly in very remote areas. Also, diagnosis of the disease is difficult and health workers may not associate such general symptoms with trypanosomiasis. Systematic screening allows early-stage disease to be detected and treated before the disease progresses, and removes the potential human reservoir. A single case of sexual transmission of West African sleeping sickness has been reported.
Treatment
First stage
The treatment for first-stage disease is fexinidazole by mouth or pentamidine by injection for T. b. gambiense. Suramin by injection is used for T. b. rhodesiense.
Second stage
Fexinidazole may be used for the second stage of TbG, if the disease is not severe. Otherwise a regimen involving the combination of nifurtimox and eflornithine, nifurtimox-eflornithine combination treatment (NECT), or eflornithine alone appear to be more effective and result in fewer side effects. These treatments may replace melarsoprol when available. NECT has the benefit of requiring less injections of eflornithine.
Intravenous melarsoprol was previously the standard treatment for second-stage (neurological phase) disease and is effective for both types. Melarsoprol is the only treatment for second stage T. b. rhodesiense; however, it causes death in 5% of people who take it. Resistance to melarsoprol can occur.
Prognosis
If untreated, T. b. gambiense almost always results in death, with only a few individuals shown in a long-term 15 year follow-up to have survived after refusing treatment. T. b. rhodesiense, being a more acute and severe form of the disease, is consistently fatal if not treated. Disease progression greatly varies depending on disease form. For individuals which are infected by T. b. gambiense, which accounts for 98% of all of the reported cases, a person can be infected for months or even years without signs or symptoms until the advanced disease stage, where it is too late to be treated successfully. For individuals affected by T. b. rhodesiense, which accounts for 2% of all reported cases, symptoms appear within weeks or months of the infection. Disease progression is rapid and invades the central nervous system, causing death within a short amount of time.
Epidemiology
In 2010, it caused around 9,000 deaths, down from 34,000 in 1990. As of 2000, the disability-adjusted life-years (9 to 10 years) lost due to sleeping sickness are 2.0 million. From 2010 to 2014, there was an estimated 55 million people at risk for gambiense African Trypanosomiasis and over 6 million people at risk for rhodesiense African trypanosomiasis. In 2014, the World Health Organization reported 3,797 cases of Human African Trypanosomiasis when the predicted number of cases were to be 5,000. The number of total reported cases in 2014 is an 86% reduction to the total number of cases reported in 2000.
The disease has been recorded as occurring in 37 countries, all in sub-Saharan Africa. It occurs regularly in southeast Uganda and western Kenya, and killed more than 48,000 Africans in 2008. The Democratic Republic of the Congo is the most affected country in the world, accounting for 75% of the Trypanosoma brucei gambiense cases. The population at risk being about 69 million with one third of this number being at a 'very high' to 'moderate' risk and the remaining two thirds at a 'low' to 'very low' risk. The number of people being affected by the disease has declined. At this rate, sleeping sickness elimination is a possibility. The World Health Organization plans to eradicate sleeping sickness by 2030.
![Chancre of human African trypanosomiasis[13]](https://upload.wikimedia.org/wikipedia/commons/thumb/e/ee/PMC5373517_pntd.0005324.g001.png/200px-PMC5373517_pntd.0005324.g001.png)
![Typical fine-spotted pink rash of acute African trypanosomiasis on the skin of the abdomen ("trypanid rash")[14]](https://upload.wikimedia.org/wikipedia/commons/thumb/8/8e/AcuteSleepingSickness.jpg/200px-AcuteSleepingSickness.jpg)
![Numerous spots of bleeding into the skin of the leg in a person infected with T. b. rhodesiense[14]](https://upload.wikimedia.org/wikipedia/commons/thumb/0/01/SSHemorragicRash.jpg/191px-SSHemorragicRash.jpg)