| Infectious mononucleosis | |
|---|---|
| Other names | Glandular fever, Pfeiffer's disease, Filatov's disease, kissing disease |
 | |
| Swollen lymph nodes in the neck of a person with infectious mononucleosis | |
| Specialty | Infectious disease |
| Symptoms | Fever, sore throat, enlarged lymph nodes in the neck, tiredness |
| Complications | Swelling of the liver or spleen |
| Duration | 2–4 weeks |
| Causes | Epstein–Barr virus (EBV) usually spread via saliva |
| Diagnostic method | Based on symptoms and blood tests |
| Treatment | Drinking enough fluids, getting sufficient rest, pain medications such as paracetamol (acetaminophen) and ibuprofen |
| Frequency | 45 per 100,000 per year (USA) |
Infectious mononucleosis (IM, mono), also known as glandular fever, is an infection usually caused by the Epstein–Barr virus (EBV). Most people are infected by the virus as children, when the disease produces few or no symptoms. In young adults, the disease often results in fever, sore throat, enlarged lymph nodes in the neck, and tiredness. Most people recover in two to four weeks; however, feeling tired may last for months. The liver or spleen may also become swollen, and in less than one percent of cases splenic rupture may occur.
While usually caused by Epstein–Barr virus, also known as human herpesvirus 4, which is a member of the herpes virus family, a few other viruses may also cause the disease. It is primarily spread through saliva but can rarely be spread through semen or blood. Spread may occur by objects such as drinking glasses or toothbrushes. Those who are infected can spread the disease weeks before symptoms develop. Mono is primarily diagnosed based on the symptoms and can be confirmed with blood tests for specific antibodies. Another typical finding is increased blood lymphocytes of which more than 10% are atypical. The monospot test is not recommended for general use due to poor accuracy.
There is no vaccine for EBV, but infection can be prevented by not sharing personal items or saliva with an infected person. Mono generally improves without any specific treatment. Symptoms may be reduced by drinking enough fluids, getting sufficient rest, and taking pain medications such as paracetamol (acetaminophen) and ibuprofen.
Mono most commonly affects those between the ages of 15 to 24 years in the developed world. In the developing world, people are more often infected in early childhood when there are fewer symptoms. In those between 16 and 20 it is the cause of about 8% of sore throats. About 45 out of 100,000 people develop infectious mono each year in the United States. Nearly 95% of people have had an EBV infection by the time they are adults. The disease occurs equally at all times of the year. Mononucleosis was first described in the 1920s and colloquially known as "the kissing disease".
Contents
Signs and symptoms
Main symptoms of infectious mononucleosis
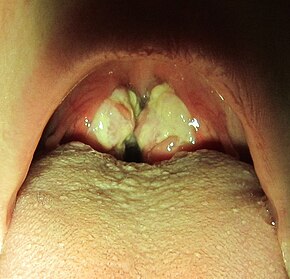
Exudative pharyngitis in a person with infectious mononucleosis
Children
Before puberty, the disease typically only produces flu-like symptoms, if any at all. When found, symptoms tend to be similar to those of common throat infections (mild pharyngitis, with or without tonsillitis).
Adolescents and young adults
In adolescence and young adulthood, the disease presents with a characteristic triad:
- Fever – usually lasting 14 days; often mild
- Sore throat – usually severe for 3–5 days, before resolving in the next 7–10 days.
- Swollen glands – mobile; usually located around the back of the neck (posterior cervical lymph nodes) and sometimes throughout the body.
Another major symptom is feeling tired. Headaches are common, and abdominal pains with nausea or vomiting sometimes also occur. Symptoms most often disappear after about 2–4 weeks. However, fatigue and a general feeling of being unwell (malaise) may sometimes last for months. Fatigue lasts more than one month in an estimated 28% of cases. Mild fever, swollen neck glands and body aches may also persist beyond 4 weeks. Most people are able to resume their usual activities within 2–3 months.
The most prominent sign of the disease is often the pharyngitis, which is frequently accompanied by enlarged tonsils with pus—an exudate similar to that seen in cases of strep throat. In about 50% of cases, small reddish-purple spots called petechiae can be seen on the roof of the mouth. Palatal enanthem can also occur, but is relatively uncommon.
A small minority of people spontaneously present a rash, usually on the arms or trunk, which can be macular (morbilliform) or papular. Almost all people given amoxicillin or ampicillin eventually develop a generalized, itchy maculopapular rash, which however does not imply that the person will have adverse reactions to penicillins again in the future. Occasional cases of erythema nodosum and erythema multiforme have been reported. Seizures may also occasionally occur.
Complications
Spleen enlargement is common in the second and third weeks, although this may not be apparent on physical examination. Rarely the spleen may rupture. There may also be some enlargement of the liver. Jaundice occurs only occasionally.
It generally gets better on its own in people who are otherwise healthy. When caused by EBV, infectious mononucleosis is classified as one of the Epstein-Barr virus-associated lymphoproliferative diseases. Occasionally the disease may persist and result in a chronic infection. This may develop into systemic EBV-positive T cell lymphoma.
Older adults
Infectious mononucleosis mainly affects younger adults.
When older adults do catch the disease, they less often have
characteristic signs and symptoms such as the sore throat and
lymphadenopathy. Instead, they may primarily experience prolonged fever, fatigue, malaise and body pains. They are more likely to have liver enlargement and jaundice. People over 40 years of age are more likely to develop serious illness.
Incubation period
The exact length of time between infection and symptoms is unclear. A review of the literature made an estimate of 33–49 days. In adolescents and young adults, symptoms are thought to appear around 4–6 weeks after initial infection. Onset is often gradual, though it can be abrupt. The main symptoms may be preceded by 1–2 weeks of fatigue, feeling unwell and body aches.
Cause
Epstein–Barr virus
About 90% of cases of infectious mononucleosis are caused by the Epstein–Barr virus, a member of the Herpesviridae family of DNA viruses. It is one of the most commonly found viruses
throughout the world. Contrary to common belief, the Epstein–Barr virus
is not highly contagious. It can only be contracted through direct
contact with an infected person's saliva, such as through kissing or sharing toothbrushes.
About 95% of the population has been exposed to this virus by the age
of 40, but only 15–20% of teenagers and about 40% of exposed adults
actually become infected.
Cytomegalovirus
A minority of cases of infectious mononucleosis is caused by human cytomegalovirus (CMV), another type of herpes virus. This virus is found in body fluids including saliva, urine, blood, and tears. A person becomes infected with this virus
by direct contact with infected body fluids. Cytomegalovirus is most
commonly transmitted through kissing and sexual intercourse. It can also
be transferred from an infected mother to her unborn child. This virus
is often "silent" because the signs and symptoms cannot be felt by the
person infected. However, it can cause life-threatening illness in infants, people with HIV, transplant recipients, and those with weak immune systems. For those with weak immune systems, cytomegalovirus can cause more serious illnesses such as pneumonia and inflammations of the retina, esophagus, liver, large intestine, and brain.
Approximately 90% of the human population has been infected with
cytomegalovirus by the time they reach adulthood, but most are unaware
of the infection. Once a person becomes infected with cytomegalovirus, the virus stays in his/her body fluids throughout the person's lifetime.
Transmission
Epstein–Barr virus infection is spread via saliva, and has an incubation period of four to seven weeks. The length of time that an individual remains contagious
is unclear, but the chances of passing the illness to someone else may
be the highest during the first six weeks following infection. Some
studies indicate that a person can spread the infection for many months,
possibly up to a year and a half.
Pathophysiology
The virus replicates first within epithelial cells in the pharynx (which causes pharyngitis, or sore throat), and later primarily within B cells (which are invaded via their CD21). The host immune response involves cytotoxic (CD8-positive) T cells against infected B lymphocytes, resulting in enlarged, atypical lymphocytes (Downey cells).
When the infection is acute (recent onset, instead of chronic), heterophile antibodies are produced.
Cytomegalovirus, adenovirus and Toxoplasma gondii (toxoplasmosis) infections can cause symptoms similar to infectious mononucleosis, but a heterophile antibody test will test negative and differentiate those infections from infectious mononucleosis.
Mononucleosis is sometimes accompanied by secondary cold agglutinin disease, an autoimmune disease in which abnormal circulating antibodies directed against red blood cells can lead to a form of autoimmune hemolytic anemia. The cold agglutinin detected is of anti-i specificity.
Diagnosis
Infectious mononucleosis, peripheral smear, high power showing reactive lymphocytes
Splenomegaly due to mononucleosis resulting in a subcapsular hematoma
Splenomegaly due to mononucleosis resulting in a subcapsular hematoma
Diagnostic modalities for infectious mononucleosis include:
- Person's age, with highest risk at 10 to 30 years.
- Medical history, such as close contact with other people with infectious mononucleosis, and the presence and time of onset of "mononucleosis-like symptoms" such as fever and sore throat
- Physical examination, including palpation of any enlarged lymph nodes in the neck, or enlarged spleen.
- The heterophile antibody test is a screening test that gives results within a day, but has significantly less than full sensitivity (70–92%) in the first two weeks after clinical symptoms begin.
- Serological tests take longer time than the heterophile antibody test, but are more accurate.
Physical examination
The presence of an enlarged spleen, and swollen posterior cervical, axillary, and inguinal lymph nodes
are the most useful to suspect a diagnosis of infectious mononucleosis.
On the other hand, the absence of swollen cervical lymph nodes and
fatigue are the most useful to dismiss the idea of infectious
mononucleosis as the correct diagnosis. The insensitivity of the
physical examination in detecting an enlarged spleen means it should not
be used as evidence against infectious mononucleosis. A physical examination may also show petechiae in the palate.
Heterophile antibody test
The heterophile antibody test works by agglutination of red blood
cells from guinea pig, sheep and horse. This test is specific but not
particularly sensitive (with a false-negative rate of as high as 25% in the first week, 5–10% in the second, and 5% in the third). About 90% of diagnosed people have heterophile antibodies by week 3, disappearing in under a year. The antibodies involved in the test do not interact with the Epstein–Barr virus or any of its antigens.
Serology
Serologic tests detect antibodies directed against the Epstein–Barr virus. Immunoglobulin G (IgG), when positive, mainly reflects a past infection, whereas immunoglobulin M
(IgM) mainly reflects a current infection. EBV-targeting antibodies can
also be classified according to which part of the virus they bind to:
- Viral capsid antigen (VCA):
- Anti-VCA IgM appear early after infection, and usually disappear within 4 to 6 weeks.
- Anti-VCA IgG appears in the acute phase of EBV infection, reaches a maximum at 2 to 4 weeks after onset of symptoms and thereafter declines slightly and persists for the rest of a person’s life.
- Early antigen (EA)
- Anti-EA IgG appears in the acute phase of illness and disappears after 3 to 6 months. It is associated with having an active infection. Yet, 20% of people may have antibodies against EA for years despite having no other sign of infection.
- EBV nuclear antigen (EBNA)
- Antibody to EBNA slowly appears 2 to 4 months after onset of symptoms and persists for the rest of a person’s life.
When negative, these tests are more accurate than the heterophile
antibody test in ruling out infectious mononucleosis. When positive,
they feature similar specificity to the heterophile antibody test.
Therefore, these tests are useful for diagnosing infectious
mononucleosis in people with highly suggestive symptoms and a negative
heterophile antibody test.
Other tests
- Epstein–Barr nuclear antigen detection. While it is not normally recognizable until several weeks into the disease, and is useful for distinguishing between a recent-onset of infectious mononucleosis and symptoms caused by a previous infection.
- Elevated hepatic transaminase levels is highly suggestive of infectious mononucleosis, occurring in up to 50% of people.
- By blood film, one diagnostic criterion for infectious mononucleosis is the presence of 50% lymphocytes with at least 10% atypical lymphocytes (large, irregular nuclei), while the person also has fever, pharyngitis, and swollen lymph nodes. The atypical lymphocytes resembled monocytes when they were first discovered, thus the term "mononucleosis" was coined.
- A fibrin ring granuloma may be present.
Differential diagnosis
About
10% of people who present a clinical picture of infectious
mononucleosis do not have an acute Epstein–Barr-virus infection. A differential diagnosis of acute infectious mononucleosis needs to take into consideration acute cytomegalovirus infection and Toxoplasma gondii
infections. Because their management is much the same, it is not always
helpful, or possible, to distinguish between Epstein–Barr-virus
mononucleosis and cytomegalovirus infection. However, in pregnant women,
differentiation of mononucleosis from toxoplasmosis is important, since it is associated with significant consequences for the fetus.
Acute HIV infection
can mimic signs similar to those of infectious mononucleosis, and tests
should be performed for pregnant women for the same reason as
toxoplasmosis.
People with infectious mononucleosis are sometimes misdiagnosed
with a streptococcal pharyngitis (because of the symptoms of fever, pharyngitis and adenopathy) and are given antibiotics such as ampicillin or amoxicillin as treatment.
Other conditions from which to distinguish infectious mononucleosis include leukemia, tonsillitis, diphtheria, common cold and influenza (flu).
Treatment
Infectious mononucleosis is generally self-limiting, so only symptomatic or supportive treatments are used.
The need for rest and return to usual activities after the acute phase
of the infection may reasonably be based on the person's general energy
levels. Nevertheless, in an effort to decrease the risk of splenic rupture experts advise avoidance of contact sports and other heavy physical activity, especially when involving increased abdominal pressure or the Valsalva maneuver (as in rowing or weight training),
for at least the first 3–4 weeks of illness or until enlargement of the
spleen has resolved, as determined by a treating physician.
Medications
Paracetamol (acetaminophen) and NSAIDs, such as ibuprofen, may be used to reduce fever and pain. Prednisone, a corticosteroid, while used to try to reduce throat pain or enlarged tonsils, remains controversial due to the lack of evidence that it is effective and the potential for side effects. Intravenous corticosteroids, usually hydrocortisone or dexamethasone, are not recommended for routine use but may be useful if there is a risk of airway obstruction, a very low platelet count, or hemolytic anemia.
There is little evidence to support the use of antivirals such as aciclovir and valacyclovir although they may reduce initial viral shedding.
Although antivirals are not recommended for people with simple
infectious mononucleosis, they may be useful (in conjunction with
steroids) in the management of severe EBV manifestations, such as EBV
meningitis, peripheral neuritis, hepatitis, or hematologic
complications.
Although antibiotics exert no antiviral action they may be indicated to treat bacterial secondary infections of the throat, such as with streptococcus (strep throat). However, ampicillin and amoxicillin are not recommended during acute Epstein–Barr virus infection as a diffuse rash may develop.
Observation
Splenomegaly is a common symptom of infectious mononucleosis and health care providers may consider using abdominal ultrasonography to get insight into the enlargement of a person's spleen.
However, because spleen size varies greatly, ultrasonography is not a
valid technique for assessing spleen enlargement and should not be used
in typical circumstances or to make routine decisions about fitness for
playing sports.
Prognosis
Serious complications are uncommon, occurring in less than 5% of cases:
- CNS complications include meningitis, encephalitis, hemiplegia, Guillain–Barré syndrome, and transverse myelitis. Prior infectious mononucleosis has been linked to the development of multiple sclerosis.
- Hematologic: Hemolytic anemia (direct Coombs test is positive) and various cytopenias, and bleeding (caused by thrombocytopenia) can occur.
- Mild jaundice
- Hepatitis with the Epstein–Barr virus is rare.
- Upper airway obstruction from tonsillar hypertrophy is rare.
- Fulminant disease course of immunocompromised people are rare.
- Splenic rupture is rare.
- Myocarditis and pericarditis are rare.
- Postural orthostatic tachycardia syndrome
- Chronic fatigue syndrome
- Cancers associated with the Epstein-Barr virus include: Burkitt's lymphoma, Hodgkin's lymphoma and lymphomas in general as well as nasopharyngeal and gastric carcinoma.
Once the acute symptoms of an initial infection disappear, they often
do not return. But once infected, the person carries the virus for the
rest of their life. The virus typically lives dormantly in B
lymphocytes. Independent infections of mononucleosis may be contracted
multiple times, regardless of whether the person is already carrying the
virus dormantly. Periodically, the virus can reactivate, during which
time the person is again infectious, but usually without any symptoms of
illness.[2]
Usually, a person with IM has few, if any, further symptoms or problems
from the latent B lymphocyte infection. However, in susceptible hosts
under the appropriate environmental stressors, the virus can reactivate
and cause vague physical symptoms (or may be subclinical), and during
this phase the virus can spread to others.
History
The characteristic symptomatology of infectious mononucleosis does
not appear to have been reported until the late nineteenth century. In 1885, the renowned Russian pediatrician Nil Filatov
reported an infectious process he called "idiopathic denitis"
exhibiting symptoms that correspond to infectious mononucleosis, and in
1889 a German balneologist and pediatrician, Emil Pfeiffer,
independently reported similar cases (some of lesser severity) that
tended to cluster in families, for which he coined the term Drüsenfieber ("glandular fever").
The word mononucleosis has several senses. It can refer to any monocytosis (excessive numbers of circulating monocytes),
but today it usually is used in its narrower sense of infectious
mononucleosis, which is caused by EBV and of which monocytosis is a
finding.
The term "infectious mononucleosis" was coined in 1920 by Thomas
Peck Sprunt and Frank Alexander Evans in a classic clinical description
of the disease published in the Bulletin of the Johns Hopkins Hospital, entitled "Mononuclear leukocytosis in reaction to acute infection (infectious mononucleosis)".
A lab test for infectious mononucleosis was developed in 1931 by Yale
School of Public Professor John Rodman Paul and Walls Willard Bunnell
based on their discovery of heterophile antibodies in the sera of
persons with the disease. The Paul-Bunnell Test or PBT was later replaced by the heterophile antibody test.
The Epstein–Barr virus was first identified in Burkitt's lymphoma cells by Michael Anthony Epstein and Yvonne Barr at the University of Bristol in 1964. The link with infectious mononucleosis was uncovered in 1967 by Werner and Gertrude Henle at the Children's Hospital of Philadelphia,
after a laboratory technician handling the virus contracted the
disease: comparison of serum samples collected from the technician
before and after the onset revealed development of antibodies to the virus.
Yale School of Public Health epidemiologist Alfred E. Evans
confirmed through testing that mononucleosis was transmitted mainly
through kissing leading to it being referred to colloquially as "the
kissing disease".