From Wikipedia, the free encyclopedia
Genetically modified crops (GMCs, GM crops, or biotech crops) are plants used in agriculture, the DNA of which has been modified using genetic engineering techniques. In most cases the aim is to introduce a new trait to the plant which does not occur naturally in the species. Examples in food crops include resistance to certain pests, diseases, or environmental conditions, reduction of spoilage, or resistance to chemical treatments (e.g. resistance to a herbicide), or improving the nutrient profile of the crop. Examples in non-food crops include production of pharmaceutical agents, biofuels, and other industrially useful goods, as well as for bioremediation.
Farmers have widely adopted GM technology. Between 1996 and 2011, the total surface area of land cultivated with GM crops had increased by a factor of 94, from 17,000 square kilometers (4,200,000 acres) to 1,600,000 km2 (395 million acres). 10% of the world's crop lands were planted with GM crops in 2010. As of 2011, 11 different transgenic crops were grown commercially on 395 million acres (160 million hectares) in 29 countries.
There is broad scientific consensus that food on the market derived from GM crops poses no greater risk to human health than conventional food.[1][2][3][4][5] GM crops also provide a number of ecological benefits.[6] However, opponents have objected to GM crops per se on several grounds, including environmental concerns, whether food produced from GM crops is safe, whether GM crops are needed to address the world's food needs, and economic concerns raised by the fact these organisms are subject to intellectual property law.
Gene transfer in nature and traditional agriculture
DNA transfers naturally between organisms.[7] Several natural mechanisms allow gene flow across species. These occur in nature on a large scale – for example, it is one mechanism for the development of antibiotic resistance in bacteria.[8] This is facilitated by transposons, retrotransposons, proviruses and other mobile genetic elements that naturally translocate DNA to new loci in a genome.[9][10] Movement occurs over an evolutionary time scale[11][12][13]The introduction of foreign germplasm into crops has been achieved by traditional crop breeders by overcoming species barriers. A hybrid cereal grain was created in 1875, by crossing wheat and rye.[14] Since then important traits including dwarfing genes and rust resistance have been introduced.[15] Plant tissue culture and deliberate mutations have enabled humans to alter the makeup of plant genomes.[16][17]
History
The first genetically modified plant was produced in 1982, an antibiotic-resistant tobacco plant.[18] The first field trials occurred in France and the USA in 1986, when tobacco plants were engineered for herbicide resistance.[19] In 1987, Plant Genetic Systems (Ghent, Belgium), founded by Marc Van Montagu and Jeff Schell, was the first company to genetically engineer insect-resistant (tobacco) plants by incorporating genes that produced insecticidal proteins from Bacillus thuringiensis (Bt).[20]The People’s Republic of China was the first country to allow commercialized transgenic plants, introducing a virus-resistant tobacco in 1992,[21] which was withdrawn in 1997.[22]:3 The first genetically modified crop approved for sale in the U.S., in 1994, was the FlavrSavr tomato. It had a longer shelf life, because it took longer to soften after ripening.[23] In 1994, the European Union approved tobacco engineered to be resistant to the herbicide bromoxynil, making it the first commercially genetically engineered crop marketed in Europe.[24]
In 1995, Bt Potato was approved by the US Environmental Protection Agency, making it the country's first pesticide producing crop.[25] In 1995 canola with modified oil composition (Calgene), Bt maize (Ciba-Geigy), bromoxynil-resistant cotton (Calgene), Bt cotton (Monsanto), glyphosate-resistant soybeans (Monsanto), virus-resistant squash (Asgrow), and additional delayed ripening tomatoes (DNAP, Zeneca/Peto, and Monsanto) were approved.[19] As of mid-1996, a total of 35 approvals had been granted to commercially grow 8 transgenic crops and one flower crop (carnation), with 8 different traits in 6 countries plus the EU.[19] In 2000, Vitamin A-enriched golden rice, was the first food with increased nutrient value.
Methods

Genetically engineered crops have genes added or removed using genetic engineering techniques,[26] originally including gene guns, electroporaton, microinjection and agrobacterium. More recently, CRISPR and TALEN offered much more precise and convenient editing techniques.
Gene guns (a.k.a. biolistic) "shoot" (direct high energy particles or radiations against[27]) target genes into plant cells. It is the most common method. DNA is bound to tiny particles of gold or tungsten which are subsequently shot into plant tissue or single plant cells under high pressure. The accelerated particles penetrate both the cell wall and membranes. The DNA separates from the metal and is integrated into plant DNA inside the nucleus. This method has been applied successfully for many cultivated crops, especially monocots like wheat or maize, for which transformation using Agrobacterium tumefaciens has been less successful.[28] The major disadvantage of this procedure is that serious damage can be done to the cellular tissue.
Agrobacterium tumefaciens-mediated transformation is another common technique. Agrobacteria are natural plant parasites, and their natural ability to transfer genes provides another engineering method. To create a suitable environment for themselves, these Agrobacteria insert their genes into plant hosts, resulting in a proliferation of modified plant cells near the soil level (crown gall). The genetic information for tumour growth is encoded on a mobile, circular DNA fragment (plasmid). When Agrobacterium infects a plant, it transfers this T-DNA to a random site in the plant genome. When used in genetic engineering the bacterial T-DNA is removed from the bacterial plasmid and replaced with the desired foreign gene. The bacterium is a vector, enabling transportation of foreign genes into plants. This method works especially well for dicotyledonous plants like potatoes, tomatoes, and tobacco. Agrobacteria infection is less successful in crops like wheat and maize.
Electroporation is used when the plant tissue does not contain cell walls. In this technique, "DNA enters the plant cells through miniature pores which are temporarily caused by electric pulses."
Microinjection directly injects the gene into the DNA.[29]
Plant scientists, backed by results of modern comprehensive profiling of crop composition, point out that crops modified using GM techniques are less likely to have unintended changes than are conventionally bred crops.[30][31]
In research tobacco and Arabidopsis thaliana are the most frequently modified plants, due to well-developed transformation methods, easy propagation and well studied genomes.[32][33] They serve as model organisms for other plant species.
Introducing new genes into plants requires a promoter specific to the area where the gene is to be expressed. For instance, to express a gene only in rice grains and not in leaves, an endosperm-specific promoter is used. The codons of the gene must be optimized for the organism due to codon usage bias. Transgenic gene products should be able to be denatured by heat so that they are destroyed during cooking.
Types of modifications
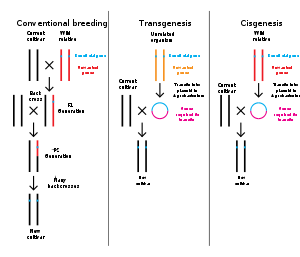
Transgenic
Transgenic plants have genes inserted into them that are derived from another species. The inserted genes can come from species within the same kingdom (plant to plant) or between kingdoms (for example, bacteria to plant). In many cases the inserted DNA has to be modified slightly in order to correctly and efficiently express in the host organism. Transgenic plants are used to express proteins like the cry toxins from B. thuringiensis, herbicide resistant genes, antibodies[34] and antigens for vaccinations[35]Transgenic carrots have been used to produce the drug Taliglucerase alfa which is used to treat Gaucher's disease.[36] In the laboratory, transgenic plants have been modified to increase photosynthesis (currently about 2% at most plants to the theoretic potential of 9–10%.[37] This is possible by changing the rubisco enzyme (i.e. changing C3 plants into C4 plants[38]), by placing the rubisco in a carboxysome, by adding CO2 pumps in the cell wall,[39][40] by changing the leaf form/size.[41][42][43][44] Plants have been engineered to exhibit bioluminescence that may become a sustainable alternative to electric lighting.[45] Still other transgenic plants have been modified to fix ambient nitrogen.[46]
Cisgenic
Cisgenic plants are made using genes found within the same species or a closely related one, where conventional plant breeding can occur. Some breeders and scientists argue that cisgenic modification is useful for plants that are difficult to crossbreed by conventional means (such as potatoes), and that plants in the cisgenic category should not require the same regulatory scrutiny as transgenics.[47]Subgenic
In 2014, Chinese researcher Gao Caixia filed patents on the creation of a strain of wheat that is resistant to powdery mildew. The strain lacks genes that encode proteins that repress defenses against the mildew. The researchers deleted all three copies of the genes from wheat's hexaploid genome. The strain promises to reduce or eliminate the heavy use of fungicides to control the disease. Gao used the TALENs and CRISPR gene editing tools without adding or changing any other genes. No field trials were immediately planned.[48][49]Business impact
The global value of biotech seed alone was US$13.2 billion in 2011, with the end product of commercial grain from biotech maize, soybean grain and cotton valued at approximately US$160 billion or more per year.[50]Participants in agriculture business markets include seed companies, agrochemical companies, distributors, farmers, grain elevators and universities that develop new crops/traits and whose agricultural extensions advise farmers on best practices.
In 2009, Monsanto had $7.3 billion in sales of seeds and from licensing its technology; DuPont, through its Pioneer subsidiary, was the next biggest company in that market.[51]
As of 2009, the overall Roundup line of products including the GM seeds represented about 50% of Monsanto's business.[52] The patent on the first type of Roundup Ready crop that Monsanto produced (soybeans) expired in 2014[53] and the first harvest of off-patent soybeans occurs in the spring of 2015.[54] Monsanto has broadly licensed the patent to other seed companies that include the glyphosate resistance trait in their seed products.[55] About 150 companies have licensed the technology,[56] including Syngenta[57] and DuPont Pioneer.[58]
Monsanto's triple-stack corn—a combination of Roundup Ready 2-weed control technology with YieldGard (Bt) Corn Borer and YieldGard Rootworm insect control—is the US market leader. U.S. corn farmers planted more than 32 million acres (130,000 km2) of triple-stack corn in 2008.[59] It is estimated that it could be planted on 56 million acres (230,000 km2) in 2014–2015. Bollgard II cotton with Roundup Ready Flex was planted on approximately 5 million acres (20,000 km2) of U.S. cotton in 2008.[60]
According to the International Service for the Acquisition of Agri-Biotech Applications (ISAAA), in 2010 approximately 15 million farmers grew biotech crops in 29 countries. Over 90% of the farmers were resource-poor in developing countries.[61] 6.5 million farmers in China and 6.3 million small farmers in India grew biotech crops (mostly Bt cotton). The Philippines, South Africa (biotech cotton, maize, and soybeans often grown by subsistence women farmers) and another twelve developing countries also grew biotech crops in 2009.[62] 10 million more small and resource-poor farmers may have been secondary beneficiaries of Bt cotton in China.
According to a 2012 review based on data from the late 1990s and early 2000s, much of the GM crop grown each year is used for livestock feed and increased demand for meat will lead to increased demand for GM feedbcrops.[63] Feed grain usage as a percentage of total crop production is 70% for corn and more than 90% of oil seed meals such as soybeans. About 65 million metric tons of GM corn grains and about 70 million metric tons of soybean meals derived from GM soybean become feed.[63]
Yield
In 2014 the largest review yet concluded that GM crops’ effects on farming were positive. The meta-analysis considered all published English-language examinations of the agronomic and economic impacts between 1995 and March 2014. The study found that herbicide-tolerant crops have lower production costs, while for insect-resistant crops the reduced pesticide use was offset by higher seed prices, leaving overall production costs about the same.[64][65]Yields increased 9% for herbicide tolerance and 25% for insect resistance. Farmers who adopted GM crops made 69% higher profits than those who did not. The review found that GM crops help farmers in developing countries, increasing yields by 14 percentage points.[64]
The researchers considered some studies that were not peer-reviewed, and a few that did not report sample sizes. They attempted to correct for publication bias, by considering sources beyond academic journals. The large data set allowed the study to control for potentially confounding variables such as fertiliser use. Separately, they concluded that the funding source did not influence study results.[64]
Traits
GM crops grown today, or under development, have been modified with various traits. These traits include improved shelf life, disease resistance, stress resistance, herbicide resistance, pest resistance, production of useful goods such as biofuel or drugs, and ability to absorb toxins and for use in bioremediation of pollution.Recently, research and development has been targeted to enhancement of crops that are locally important in developing countries, such as insect-resistant cowpea for Africa[66] and insect-resistant brinjal (eggplant) for India.[67]
Lifetime
The first genetically modified crop approved for sale in the U.S. was the FlavrSavr tomato, which had a longer shelf life.[23] It is no longer on the market.In November 2014, the USDA approved a GM potato that prevents bruising.[68][69]
In February 2015 Arctic Apples were approved by the USDA,[70] becoming the first genetically modified apple approved for US sale.[71] Gene silencing is used to reduce the expression of polyphenol oxidase (PPO), thus preventing enzymatic browning of the exposed fruit after it has been sliced open. The trait was added to Granny Smith and Golden Delicious varieties.[70][72] The trait also includes a bacterial antibiotic gene that provides resistance to the antibiotic kanamycin. The modification process affected only a small fraction of processed cells. The cells were then cultivated in the presence of kanamycin, which allows only resistant cultivars to survive. Consuming resistant apples does not provide kanamycin resistance.[73] As of the announcement, the FDA continued to review the strains.[71]
Nutrition
Edible oils
Some GM soybeans offer improved oil profiles for processing or healthier eating.[74][75] Camelina sativa has been modified to produce plants that accumulate high levels of oils similar to fish oils.[76][77]Vitamin enrichment
Golden rice, developed by the International Rice Research Institute (IRRI), provides greater amounts of Vitamin A targeted at reducing Vitamin A deficiency.[78][79]Researchers vitamin-enriched corn derived from South African white corn variety M37W, producing a 169-fold increase in Vitamin A, 6-fold increase in Vitamin C and doubled concentrations of folate.[80] Modified Cavendish bananas express 10-fold the amount of Vitamin A as unmodified varieties.[81]
Toxin reduction
A genetically modified cassava under development offers lower cyanogen glucosides and enhanced protein and other nutrients (called BioCassava).[82]In November 2014, the USDA approved a potato, developed by J.R. Simplot Company, that prevents bruising and produces less acrylamide when fried. The modifications prevent natural, harmful proteins from being made via RNA interference.[68][69] They do not employ genes from non-potato species. The trait was added to the Russet Burbank, Ranger Russet and Atlantic varieties.[68]
Stress resistance
Plants engineered to tolerate non-biological stressors such as drought,[68][69][83][84] frost,[85][86] high soil salinity,[87][88] and nitrogen starvation[89] were in development. In 2011, Monsanto's DroughtGard maize became the first drought-resistant GM crop to receive US marketing approval.[90]Herbicides
Glyphosate
As of 1999 the most prevalent GM trait was glyphosate-resistance.[91] Glyphosate, (the active ingredient in Roundup and other herbicide products) kills plants by interfering with the shikimate pathway in plants, which is essential for the synthesis of the aromatic amino acids phenylalanine, tyrosine and tryptophan. The shikimate pathway is not present in animals, which instead obtain aromatic amino acids from their diet. More specifically, glyphosate inhibits the enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS).This trait was developed because the herbicides used on grain and grass crops at the time were highly toxic and not effective against narrow-leaved weeds. Thus, developing crops that could withstand spraying with glyphosate would both reduce environmental and health risks, and give an agricultural edge to the farmer.[91]
Some micro-organisms have a version of EPSPS that is resistant to glyphosate inhibition. One of these was isolated from an Agrobacterium strain CP4 (CP4 EPSPS) that was resistant to glyphosate.[92][93] The CP4 EPSPS gene was engineered for plant expression by fusing the 5' end of the gene to a chloroplast transit peptide derived from the petunia EPSPS. This transit peptide was used because it had shown previously an ability to deliver bacterial EPSPS to the chloroplasts of other plants. This CP4 EPSPS gene was cloned and transfected into soybeans.
The plasmid used to move the gene into soybeans was PV-GMGTO4. It contained three bacterial genes, two CP4 EPSPS genes, and a gene encoding beta-glucuronidase (GUS) from Escherichia coli as a marker. The DNA was injected into the soybeans using the particle acceleration method. Soybean cultivar A54O3 was used for the transformation.
Bromoxynil
Tobacco plants have been engineered to be resistant to the herbicide bromoxynil.[24]Glufosinate
Crops have been commercialized that are resistant to the herbicide glufosinate, as well.[94] Crops engineered for resistance to multiple herbicides to allow farmers to use a mixed group of two, three, or four different chemicals are under development to combat growing herbicide resistance.[95][96]2-4D
In October 2014 the US EPA registered Dow's "Enlist Duo" maize, which is genetically modified to be resistant to both glyphosate and 2,4-D, in six states.[97][98] The genetic modification providing resistance to 2,4-D is insertiion of a bacterial aryloxyalkanoate dioxygenase gene, aad1.[97][99] The USDA had approved maize and soybeans with the mutation in September 2014.[100]Dicamba
Monsanto has requested approval for a stacked strain that is tolerant of both glyphosate and dicamba.[101]Pest resistance
Insects
Tobacco, corn, rice and many other crops have been engineered to express genes encoding for insecticidal proteins from Bacillus thuringiensis (Bt).[25][102] Papaya, potatoes, and squash have been engineered to resist viral pathogens such as cucumber mosaic virus which, despite its name, infects a wide variety of plants.[103]In the late 1990s, a GM potato that was resistant to the Colorado potato beetle was withdrawn because major buyers rejected it, fearing consumer opposition.[68]
Viruses
Virus resistant papaya were developed In response to a papaya ringspot virus (PRV) outbreak in Hawaii in the late 1990s. . They incorporate PRV DNA.[104][105] By 2010, 80% of Hawaiian papaya plants were genetically modified.[106][107]Potatoes were engineered for resistance to potato leaf roll virus and Potato virus Y in 1998. Poor sales led to their market withdrawal after three years.[108]
Yellow squash that were resistant to at first two, then three viruses were developed, beginning in the 1990s. The viruses are watermelon, cucumber and zucchini/courgette yellow mosaic. Squash was the second GM crop to be approved by US regulators. The trait was later added to zucchini.[109]
By-products
Drugs
In 2012, the FDA approved the first plant-produced pharmaceutical, a treatment for Gaucher's Disease.[110] Tobacco plants have been modified to produce therapeutic antibodies.[111]Biofuel
Algae is under development for use in biofuels.[112] Modified jatropha offers improved qualities for fuel. Syngenta has USDA approval to market a maize trademarked Enogen that has been genetically modified to convert its starch to sugar for ethanol.[113] In 2013, the Flemish Institute for Biotechnology was investigating poplar trees genetically engineered to contain less lignin to ease conversion into ethanol.[114] Lignin is the critical limiting factor when using wood to make bio-ethanol because lignin limits the accessibility of cellulose microfibrils to depolymerization by enzymes.[115]Materials
Companies and labs are working on plants that can be used to make bioplastics.[116] Potatoes that produce industrially useful starches have been developed as well.[117] Oilseed can be modified to produce fatty acids for detergents, substitute fuels and petrochemicals.Bioremediation
Scientists at the University of York developed a weed (Arabidopsis thaliana) that contains genes from bacteria that can clean TNT and RDX-explosive soil contaminants.[118] 16 million hectares in the USA (1.5% of the total surface) are estimated to be contaminated with TNT and RDX. However A. thaliana was not tough enough for use on military test grounds.[119]Genetically modified plants have been used for bioremediation of contaminated soils. Mercury, selenium and organic pollutants such as polychlorinated biphenyls (PCBs).[119][120]
Marine environments are especially vulnerable since pollution such as oil spills are not containable. In addition to anthropogenic pollution, millions of tons of petroleum annually enter the marine environment from natural seepages. Despite its toxicity, a considerable fraction of petroleum oil entering marine systems is eliminated by the hydrocarbon-degrading activities of microbial communities. Particularly successful is a recently discovered group of specialists, the so-called hydrocarbonoclastic bacteria (HCCB) that may offer useful genes.[121]
Asexual reproduction
Crops such as maize reproduce sexually each year. This randomizes which genes get propagated to the next generation, meaning that desirable traits can be lost. To maintain a high-quality crop, some farmers purchase seeds every year. Typically, the seed company maintains two inbred varieties, and crosses them into a hybrid strain that is then sold. Related plants like sorghum and gamma grass are able to perform apomixis, a form of asexual reproduction that keeps the plant's DNA intact. This trait is apparently controlled by a single dominant gene, but traditional breeding has been unsuccessful in creating asexually-reproducing maize. Genetic engineering offers another route to this goal. Successful modification would allow farmers to replant harvested seeds that retain desirable traits, rather than relying on purchased seed.[122]Crops
As of 2010 food species for which a genetically modified version is being commercially grown (percent modified in the table below are mostly 2009/2010 data) include:[123][124][125][126][127][128]| Crop | Traits | Modification[specify] | Percent modified in US | Percent modified in world |
|---|---|---|---|---|
| Alfalfa | Tolerance of glyphosate or glufosinate | Genes added | Planted in the US from 2005–2007; 2007–2010 court injunction; 2011 approved for sale | |
| Apples | Delayed browning [72] | Genes added for reduced polyphenol oxidase (PPO production from other apples[72] | 2015 approved for sale[70] | |
| Canola/ Rapeseed | Tolerance of glyphosate or glufosinate High laurate canola,[129] Oleic acid canola[130] | Genes added | 87% (2005)[128] | 21% |
| Corn | Tolerance of herbicides glyphosate glufosinate, and 2,4-D. Insect resistance. Added enzyme, alpha amylase, that converts starch into sugar to facilitate ethanol production.[131] | Genes, some from Bt, added.[132] | Herbicide-resistant: 2013, 85%[133] Bt: 2013, 76%[133] Stacked: 2013, 71% | 26% |
| Cotton (cottonseed oil) | Insect resistance | Gene, some from Bt, added | Herbicide-resistant: 2013, 82%[133] Bt: 2013, 75%[133] Stacked: 2013, 71%[133] | 49% |
| Papaya (Hawaiian) | Resistance to the papaya ringspot virus.[134] | Gene added | 80% | |
| Potato (food) | Resistance to Colorado beetle Resistance to potato leaf roll virus and Potato virus Y[117] Reduced acrylamide when fried and reduced bruising[68] |
Bt cry3A, coat protein from PVY[135] "Innate" potatoes added genetic material coding for mRNA for RNA interference[68] | 0% | 0% |
| Potato (starch) | Antibiotic resistance gene, used for selection
Better starch production[136] |
Antibiotic resistance gene from bacteria
Modifications to endogenous starch-producing enzymes |
0% | 0% |
| Rice | Enriched with beta-carotene (a source of vitamin A) | Genes from maize and a common soil microorganism.[137][138] | Forecast to be on the market in 2015 or 2016[139] | |
| Soybeans | Tolerance of glyphosate or glufosinate
Reduced saturated fats (high oleic acid);[140] Kills susceptible insect pests |
Herbicide resistant gene taken from bacteria added
Knocked out native genes that catalyze saturation Gene for one or more Bt crystal proteins added |
2013: 93%[133] | 77% |
| Squash (Zucchini/Courgette) | Resistance to watermelon, cucumber and zucchini/courgette yellow mosaic viruses[130][141][142] | Viral coat protein genes | 13% (figure is from 2005)[128] | |
| Sugar beet | Tolerance of glyphosate, glufosinate | Genes added | 95% (2010); regulated 2011; deregulated 2012 | 9% |
| Sugarcane | Pesticide tolerance
High sucrose content. |
Genes added | ||
| Sweet peppers | Resistance to cucumber mosaic virus[143][144] | Viral coat protein genes | Small quantities grown in China | |
| Tomatoes | Suppression of the enzyme polygalacturonase (PG), retarding fruit softening after harvesting,[145] while at the same time retaining both the natural color and flavor of the fruit | Antisense gene of the gene responsible for PG enzyme production added | Taken off the market due to commercial failure. | Small quantities grown in China |
| Wheat | Tolerance of glyphosate | Genes added | unknown | unknown |
Development
The number of USDA-approved field releases for testing grew from 4 in 1985 to 1,194 in 2002 and averaged around 800 per year thereafter. The number of sites per release and the number of gene constructs (ways that the gene of interest is packaged together with other elements)—have rapidly increased since 2005. Releases with agronomic properties (such as drought resistance) jumped from 1,043 in 2005 to 5,190 in 2013. As of September 2013, about 7,800 releases had been approved for corn, more than 2,200 for soybeans, more than 1,100 for cotton, and about 900 for potatoes. Releases were approved for herbicide tolerance (6,772 releases), insect resistance (4,809), product quality such as flavor or nutrition (4,896), agronomic properties like drought resistance (5,190), and virus/fungal resistance (2,616). The institutions with the most authorized field releases include Monsanto with 6,782, Pioneer/DuPont with 1,405, Syngenta with 565, and USDA’s Agricultural Research Service with 370. As of September 2013 USDA had received proposals for releasing GM rice, squash, plum, rose, tobacco, flax and chicory.[133]Farming practices
Resistance
Constant exposure to a toxin creates evolutionary pressure for pests resistant to that toxin. Overreliance on glyphosate and a reduction in the diversity of weed management practices allowed the spread of glyphosate resistance in 14 weed species/biotypes in the US.[133]One method of reducing resistance is the creation of refuges to allow nonresistant organisms to survive and maintain a susceptible population.
To reduce resistance to Bt crops, the 1996 commercialization of transgenic cotton and maize came with a management strategy to prevent insects from becoming resistant. Insect resistance management plans are mandatory for Bt crops. The aim is to encourage a large population of pests so that any (recessive) resistance genes are diluted within the population. Resistance lowers evolutionary fitness in the absence of the stressor (Bt). In refuges, non-resistant strains outcompete resistant ones.[146]
With sufficiently high levels of transgene expression, nearly all of the heterozygotes (S/s), i.e., the largest segment of the pest population carrying a resistance allele, will be killed before maturation, thus preventing transmission of the resistance gene to their progeny.[147] Refuges (i. e., fields of nontransgenic plants) adjacent to transgenic fields increases the likelihood that homozygous resistant (s/s) individuals and any surviving heterozygotes will mate with susceptible (S/S) individuals from the refuge, instead of with other individuals carrying the resistance allele. As a result, the resistance gene frequency in the population remains lower.
Complicating factors can affect the success of the high-dose/refuge strategy. For example if the temperature is not ideal, thermal stress can lower Bt toxin production and leave the plant more susceptible. More importantly, reduced late-season expression has been documented, possibly resulting from DNA methylation of the promoter.[148] The success of the high-dose/refuge strategy has successfully maintained the value of Bt crops, this success has depended on factors independent of management strategy, including low initial resistance allele frequencies, fitness costs associated with resistance, and the abundance of non-Bt host plants outside the refuges.[149]
Best management practices (BMPs) to control weeds may help delay resistance. BMPs include applying multiple herbicides with different modes of action, rotating crops, planting weed-free seed, scouting fields routinely, cleaning equipment to reduce the transmission of weeds to other fields, and maintaining field borders.[133]
Companies that produce Bt seed are introducing strains with multiple Bt proteins. Monsanto did this with Bt cotton in India, where the product was rapidly adopted.[150]
Plant protection
Farmers generally use less insecticide when they plant Bt-resistant crops. Insecticide use on corn farms declined from 0.21 pound per planted acre in 1995 to 0.02 pound in 2010. This is consistent with the decline in European corn borer populations as a direct result of Bt corn and cotton. The establishment of minimum refuge requirements helped delay the evolution of Bt resistance. However resistance appears to be developing to some Bt traits in some areas.[133]Tillage
By leaving at least 30% of crop residue on the soil surface from harvest through planting, conservation tillage reduces soil erosion from wind and water, increases water retention, and reduces soil degradation as well as water and chemical runoff. In addition, conservation tillage reduces the carbon footprint of agriculture.[151]A 2014 review covering 12 states from 1996 to 2006, found that a 1% increase in herbicde-tolerant (HT) soybean adoption leads to a 0.21% increase in conservation tillage and a 0.3% decrease in quality-adjusted herbicide use.[151]
Regulation
The regulation of genetic engineering concerns the approaches taken by governments to assess and manage the risks associated with the development and release of genetically modified crops. There are differences in the regulation of GM crops between countries, with some of the most marked differences occurring between the USA and Europe.
Regulation varies in a given country depending on the intended use of each product. For example, a crop not intended for food use is generally not reviewed by authorities responsible for food safety.[152][153]
The United States Department of Agriculture (USDA) reports every year on the total area of GMO varieties planted in the United States.[156][157] According to National Agricultural Statistics Service, the states published in these tables represent 81–86 percent of all corn planted area, 88–90 percent of all soybean planted area, and 81–93 percent of all upland cotton planted area (depending on the year).
Global estimates are produced by the International Service for the Acquisition of Agri-biotech Applications (ISAAA) and can be found in their annual reports, "Global Status of Commercialized Transgenic Crops".[154][158]
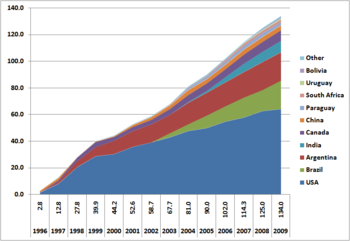
Farmers have widely adopted GM technology (see figure). Between 1996 and 2013, the total surface area of land cultivated with GM crops increased by a factor of 100, from 17,000 square kilometers (4,200,000 acres) to 1,750,000 km2 (432 million acres).[154] 10% of the world's croplands were planted with GM crops in 2010.[50] As of 2011, 11 different transgenic crops were grown commercially on 395 million acres (160 million hectares) in 29 countries such as the USA, Brazil, Argentina, India, Canada, China, Paraguay, Pakistan, South Africa, Uruguay, Bolivia, Australia, Philippines, Myanmar, Burkina Faso, Mexico and Spain.[50] One of the key reasons for this widespread adoption is the perceived economic benefit the technology brings to farmers. For example, the system of planting glyphosate-resistant seed and then applying glyphosate once plants emerged provided farmers with the opportunity to dramatically increase the yield from a given plot of land, since this allowed them to plant rows closer together. Without it, farmers had to plant rows far enough apart to control post-emergent weeds with mechanical tillage.[159] Likewise, using Bt seeds means that farmers do not have to purchase insecticides, and then invest time, fuel, and equipment in applying them. However critics have disputed whether yields are higher and whether chemical use is less, with GM crops. See Genetically modified food controversies article for information.
In the US, by 2014, 94% of the planted area of soybeans, 96% of cotton and 93% of corn were genetically modified varieties.[123][124][160] Genetically modified soybeans carried herbicide-tolerant traits only, but maize and cotton carried both herbicide tolerance and insect protection traits (the latter largely Bt protein).[161] These constitute "input-traits" that are aimed to financially benefit the producers, but may have indirect environmental benefits and cost benefits to consumers. The Grocery Manufacturers of America estimated in 2003 that 70–75% of all processed foods in the U.S. contained a GM ingredient.[162]
Europe grows relatively few genetically engineered crops[163] with the exception of Spain, where one fifth of maize is genetically engineered,[164] and smaller amounts in five other countries.[165] The EU had a 'de facto' ban on the approval of new GM crops, from 1999 until 2004.[166][167] GM crops are now regulated by the EU.[168] Developing countries grew 54 percent of genetically engineered crops in 2013.[154]
In recent years GM crops expanded rapidly in developing countries. In 2013 approximately 18 million farmers grew 54% of worldwide GM crops in developing countries.[154] 2013's largest increase was in Brazil (403,000 km2 versus 368,000 km2 in 2012). GM cotton began growing in India in 2002, reaching 110,000 km2 in 2013.[154]
According to the 2013 ISAAA brief: "...a total of 36 countries (35 + EU-28) have granted regulatory approvals for biotech crops for food and/or feed use and for environmental release or planting since 1994... a total of 2,833 regulatory approvals involving 27 GM crops and 336 GM events (NB: an "event" is a specific genetic modification in a specific species) have been issued by authorities, of which 1,321 are for food use (direct use or processing), 918 for feed use (direct use or processing) and 599 for environmental release or planting. Japan has the largest number (198), followed by the U.S.A. (165, not including "stacked" events), Canada (146), Mexico (131), South Korea (103), Australia (93), New Zealand (83), European Union (71 including approvals that have expired or under renewal process), Philippines (68), Taiwan (65), Colombia (59), China (55) and South Africa (52). Maize has the largest number (130 events in 27 countries), followed by cotton (49 events in 22 countries), potato (31 events in 10 countries), canola (30 events in 12 countries) and soybean (27 events in 26 countries).[154]
Broad scientific consensus states that currently marketed GM food poses no greater risk than conventionally produced food.[1][3][169] No reports of ill effects have been documented in the human population from GM food.[4][170][171] Although GMO labeling is required in many countries, the United States Food and Drug Administration does not require labeling, nor does it recognize a distinction between approved GMO and non-GMO foods.[172]
Advocacy groups such as Greenpeace and the World Wildlife Fund claim that risks related to GM food have not been adequately examined and managed, and have questioned the objectivity of regulatory authorities and scientific bodies.
Production[edit]
In 2013, GM crops were planted in 27 countries; 19 were developing countries and 8 were developed countries. 2013 was the second year in which developing countries grew a majority (54%) of the total GM harvest. 18 million farmers grew GM crops; around 90% were small-holding farmers in developing countries.[154]| Country | 2013– GM planted area (million hectares)[155] | Biotech crops |
|---|---|---|
| USA | 70.1 | Maize, Soybean, Cotton, Canola, Sugarbeet, Alfalfa, Papaya, Squash |
| Brazil | 40.3 | Soybean, Maize, Cotton |
| Argentina | 24.4 | Soybean, Maize, Cotton |
| India | 11.0 | Cotton |
| Canada | 10.8 | Canola, Maize, Soybean, Sugarbeet |
| Total | 175.2 | ---- |
The United States Department of Agriculture (USDA) reports every year on the total area of GMO varieties planted in the United States.[156][157] According to National Agricultural Statistics Service, the states published in these tables represent 81–86 percent of all corn planted area, 88–90 percent of all soybean planted area, and 81–93 percent of all upland cotton planted area (depending on the year).
Global estimates are produced by the International Service for the Acquisition of Agri-biotech Applications (ISAAA) and can be found in their annual reports, "Global Status of Commercialized Transgenic Crops".[154][158]
Farmers have widely adopted GM technology (see figure). Between 1996 and 2013, the total surface area of land cultivated with GM crops increased by a factor of 100, from 17,000 square kilometers (4,200,000 acres) to 1,750,000 km2 (432 million acres).[154] 10% of the world's croplands were planted with GM crops in 2010.[50] As of 2011, 11 different transgenic crops were grown commercially on 395 million acres (160 million hectares) in 29 countries such as the USA, Brazil, Argentina, India, Canada, China, Paraguay, Pakistan, South Africa, Uruguay, Bolivia, Australia, Philippines, Myanmar, Burkina Faso, Mexico and Spain.[50] One of the key reasons for this widespread adoption is the perceived economic benefit the technology brings to farmers. For example, the system of planting glyphosate-resistant seed and then applying glyphosate once plants emerged provided farmers with the opportunity to dramatically increase the yield from a given plot of land, since this allowed them to plant rows closer together. Without it, farmers had to plant rows far enough apart to control post-emergent weeds with mechanical tillage.[159] Likewise, using Bt seeds means that farmers do not have to purchase insecticides, and then invest time, fuel, and equipment in applying them. However critics have disputed whether yields are higher and whether chemical use is less, with GM crops. See Genetically modified food controversies article for information.

Land area used for genetically modified crops by country (1996–2009), in millions of hectares. In 2011, the land area used was 160 million hectares, or 1.6 million square kilometers.[50]
In the US, by 2014, 94% of the planted area of soybeans, 96% of cotton and 93% of corn were genetically modified varieties.[123][124][160] Genetically modified soybeans carried herbicide-tolerant traits only, but maize and cotton carried both herbicide tolerance and insect protection traits (the latter largely Bt protein).[161] These constitute "input-traits" that are aimed to financially benefit the producers, but may have indirect environmental benefits and cost benefits to consumers. The Grocery Manufacturers of America estimated in 2003 that 70–75% of all processed foods in the U.S. contained a GM ingredient.[162]
Europe grows relatively few genetically engineered crops[163] with the exception of Spain, where one fifth of maize is genetically engineered,[164] and smaller amounts in five other countries.[165] The EU had a 'de facto' ban on the approval of new GM crops, from 1999 until 2004.[166][167] GM crops are now regulated by the EU.[168] Developing countries grew 54 percent of genetically engineered crops in 2013.[154]
In recent years GM crops expanded rapidly in developing countries. In 2013 approximately 18 million farmers grew 54% of worldwide GM crops in developing countries.[154] 2013's largest increase was in Brazil (403,000 km2 versus 368,000 km2 in 2012). GM cotton began growing in India in 2002, reaching 110,000 km2 in 2013.[154]
According to the 2013 ISAAA brief: "...a total of 36 countries (35 + EU-28) have granted regulatory approvals for biotech crops for food and/or feed use and for environmental release or planting since 1994... a total of 2,833 regulatory approvals involving 27 GM crops and 336 GM events (NB: an "event" is a specific genetic modification in a specific species) have been issued by authorities, of which 1,321 are for food use (direct use or processing), 918 for feed use (direct use or processing) and 599 for environmental release or planting. Japan has the largest number (198), followed by the U.S.A. (165, not including "stacked" events), Canada (146), Mexico (131), South Korea (103), Australia (93), New Zealand (83), European Union (71 including approvals that have expired or under renewal process), Philippines (68), Taiwan (65), Colombia (59), China (55) and South Africa (52). Maize has the largest number (130 events in 27 countries), followed by cotton (49 events in 22 countries), potato (31 events in 10 countries), canola (30 events in 12 countries) and soybean (27 events in 26 countries).[154]
Controversy
GM foods are controversial and the subject of protests, vandalism, referenda, legislation, court action and scientific disputes. The controversies involve consumers, biotechnology companies, governmental regulators, non-governmental organizations and scientists. The key areas are whether GM food should be labeled, the role of government regulators, the effect of GM crops on health and the environment, the effects of pesticide use and resistance, the impact on farmers, and their roles in feeding the world and energy production.Broad scientific consensus states that currently marketed GM food poses no greater risk than conventionally produced food.[1][3][169] No reports of ill effects have been documented in the human population from GM food.[4][170][171] Although GMO labeling is required in many countries, the United States Food and Drug Administration does not require labeling, nor does it recognize a distinction between approved GMO and non-GMO foods.[172]
Advocacy groups such as Greenpeace and the World Wildlife Fund claim that risks related to GM food have not been adequately examined and managed, and have questioned the objectivity of regulatory authorities and scientific bodies.