From Wikipedia, the free encyclopedia
A technetium injection contained in a shielded syringe
Technetium-99m is used as a
radioactive tracer and can be detected in the body by medical equipment (
gamma cameras). It is well suited to the role, because it emits readily detectable
gamma rays with a
photon energy of 140
keV (these 8.8 pm
photons are about the same wavelength as emitted by conventional X-ray diagnostic equipment) and its
half-life for gamma emission is 6.0058 hours (meaning 93.7% of it decays to
99Tc in 24 hours). The relatively "short" physical
half-life of the isotope and its
biological half-life
of 1 day (in terms of human activity and metabolism) allows for
scanning procedures which collect data rapidly but keep total patient
radiation exposure low. The same characteristics make the isotope
suitable only for diagnostic but never therapeutic use.
Technetium-99m was discovered as a product of
cyclotron bombardment of
molybdenum. This procedure produced
molybdenum-99,
a radionuclide with a longer half-life (2.75 days), which decays to
Tc-99m. At present, molybdenum-99 (Mo-99) is used commercially as the
easily transportable source of medically used Tc-99m. In turn, this
Mo-99 is usually created commercially by fission of
highly enriched uranium in aging research and material testing nuclear reactors in several countries.
History
Discovery
we discovered an isotope of great
scientific interest, because it decayed by means of an isomeric
transition with emission of a line spectrum of electrons coming from an
almost completely internally converted gamma ray transition. [actually,
only 12% of the decays are by internal conversion] (...) This was a form
of radioactive decay which had never been observed before this time.
Segrè and I were able to show that this radioactive isotope of the
element with the atomic number 43 decayed with a half-life of 6.6 h
[later updated to 6.0 h] and that it was the daughter of a 67-h [later
updated to 66 h] molybdenum parent radioactivity. This chain of decay
was later shown to have the mass number 99, and (...) the 6.6-h activity
acquired the designation ‘technetium-99m.
Later in 1940, Emilio Segrè and
Chien-Shiung Wu
published the experimental results of the analysis of fission products
of uranium-235, among which was present molybdenum-99, and detected the
6-h activity of element 43, later labelled as technetium-99m.
Early medical applications in the United States
Tc-99m remained a scientific curiosity until the 1950s when
Powell Richards
realized the potential of technetium-99m as a medical radiotracer and
promoted its use among the medical community. While Richards was in
charge of the radioisotope production at the Hot Lab Division of the
Brookhaven National Laboratory, Walter Tucker and
Margaret Greene were working on how to improve the separation process purity of the short-lived
eluted daughter product iodine-132 from
tellurium-132, its 3.2-days parent, produced in the Brookhaven Graphite Research Reactor.
They detected a trace contaminant which proved to be Tc-99m, which was
coming from Mo-99 and was following tellurium in the chemistry of the
separation process for other fission products. Based on the similarities
between the chemistry of the tellurium-iodine parent-daughter pair,
Tucker and Greene developed the first
technetium-99m generator in 1958. It was not until 1960 that Richards became the first to suggest the idea of using technetium as a medical tracer.
The first US publication to report on medical scanning of Tc-99m appeared in August 1963.
Sorensen and Archambault demonstrated that intravenously injected
carrier-free Mo-99 selectively and efficiently concentrated in the
liver, becoming an internal generator of Tc-99m. After build-up of
Tc-99m, they could visualize the liver using the 140 keV gamma ray
emission.
Worldwide expansion
The
production and medical use of Tc-99m rapidly expanded across the world
in the 1960s, benefiting from the development and continuous
improvements of the
gamma cameras.
- Americas
Between 1963 and 1966, numerous scientific studies demonstrated the use of Tc-99m as
radiotracer or diagnostic tool. As a consequence the demand for Tc-99m grew exponentially and by 1966,
Brookhaven National Laboratory was unable to cope with the demand. Production and distribution of Tc-99m generators were transferred to private companies.
"TechneKow-CS generator", the first commercial Tc-99m generator, was produced by Nuclear Consultants, Inc. (St. Louis, Missouri) and
Union Carbide Nuclear Corporation (Tuxedo, New York). From 1967 to 1984, Mo-99 was produced for
Mallinckrodt Nuclear Company at the
Missouri University Research Reactor (MURR).
Union Carbide actively developed a process to produce and separate useful isotopes like Mo-99 from mixed
fission products that resulted from the irradiation of
highly enriched uranium
(HEU) targets in nuclear reactors developed from 1968 to 1972 at the
Cintichem facility (formerly the Union Carbide Research Center built in
the Sterling forest in Tuxedo, New York (
41°14′6.88″N 74°12′50.78″W)). The Cintichem process originally used 93% highly enriched U-235 deposited as UO
2 on the inside of a cylindrical target.
At the end of the 1970s, 200,000 Ci (7.4×1015 Bq)
of total fission product radiation were extracted weekly from 20-30
reactor bombarded HEU capsules, using the so-called "Cintichem [chemical
isolation] process." The research facility with its 1961 5-MW pool-type research reactor was later sold to Hoffman-LaRoche and became Cintichem Inc.
In 1980, Cintichem, Inc. began the production/isolation of Mo-99 in its
reactor, and became the single U.S. producer of Mo-99 during the 1980s.
However, in 1989, Cintichem detected an underground leak of radioactive
products that led to the reactor shutdown and decommissioning, putting
an end to the commercial production of Mo-99 in the USA.
The production of Mo-99 started in Canada in the early 1970s and was shifted to the NRU reactor in the mid 1970s.
By 1978 the reactor provided technetium-99m in large enough quantities
that were processed by AECL's radiochemical division, which was
privatized in 1988 as Nordion, now
MDS Nordion. In the 1990s a substitution for the aging NRU reactor for production of radioisotopes was planned. The
Multipurpose Applied Physics Lattice Experiment (MAPLE) was designed as a dedicated isotope-production facility. Initially, two identical MAPLE reactors were to be built at
Chalk River Laboratories,
each capable of supplying 100% of the world's medical isotope demand.
However, problems with the MAPLE 1 reactor, most notably a positive
power co-efficient of reactivity, led to the cancellation of the project in 2008.
The first commercial Tc-99m generators were produced in
Argentina in 1967, with Mo-99 produced in the
CNEA's
RA-1 Enrico Fermi reactor. Besides its domestic market CNEA supplies Mo-99 to some South American countries.
- Asia
In 1967, the first Tc-99m procedures were carried out in
Auckland,
New Zealand. Mo-99 was initially supplied by Amersham, UK, then by the Australian Nuclear Science and Technology Organisation (
ANSTO) in Lucas Heights, Australia.
- Europe
In May 1963, Scheer and Maier-Borst were the first to introduce the use of Tc-99m for medical applications.
In 1968,
Philips-Duphar (later Mallinckrodt, today
Covidien) marketed the first technetium-99m generator produced in Europe and distributed from Petten, the Netherlands.
Shortage
Global shortages of technetium-99m emerged in the late 2000s because two aging nuclear reactors (
NRU and
HFR)
that provided about two-thirds of the world’s supply of molybdenum-99,
which itself has a half-life of only 66 hours, were shut down repeatedly
for extended maintenance periods. In May 2009 the
Atomic Energy of Canada Limited announced the detection of a small leak of
heavy water
in the NRU reactor that remained out of service until completion of the
repairs in August 2010. After the observation of gas bubble jets
released from one of the deformations of primary cooling water circuits
in August 2008, the HFR reactor was stopped for a thorough safety
investigation.
NRG
received in February 2009 a temporary license to operate HFR only when
necessary for medical radioisotope production. HFR stopped for repairs
at the beginning of 2010 and was restarted in September 2010.
Two replacement Canadian reactors constructed in the 1990s were closed before beginning operation, for safety reasons. A construction permit for a new production facility to be built in
Columbia, MO was issued in May 2018.
Nuclear properties
Tc-99m decays mainly by gamma emission, slightly less than 88% of the time. (
99mTc →
99Tc
+ γ) About 98.6% of these gamma decays result in 140.5 keV gamma rays
and the remaining 1.4% are to gammas of a slightly higher energy at
142.6 keV. These are the radiations that are picked up by a gamma camera
when
99mTc is used as a
radioactive tracer for
medical imaging. The remaining approximately 12% of
99mTc decays are by means of
internal conversion,
resulting in ejection of high speed internal conversion electrons in
several sharp peaks (as is typical of electrons from this type of decay)
also at about 140 keV (
99mTc →
99Tc
+ + e
−). These conversion electrons will
ionize the surrounding matter like
beta radiation electrons would do, contributing along with the 140.5 keV and 142.6 keV gammas to the total deposited
dose.
Pure gamma emission is the desirable
decay mode for medical imaging because other particles deposit more energy in the patient body (
radiation dose) than in the camera. Metastable isomeric transition is the only nuclear decay mode that approaches pure gamma emission.
Tc-99m's
half-life
of 6.0058 hours is considerably longer (by 14 orders of magnitude, at
least) than most nuclear isomers, though not unique. This is still a
short half-life relative to many other known modes of
radioactive decay and it is in the middle of the range of half lives for
radiopharmaceuticals used for
medical imaging.
After gamma emission or internal conversion, the resulting
ground-state technetium-99 then decays with a half-life of 211,000 years
to
stable ruthenium-99.
This process emits soft beta radiation without a gamma. Such low
radioactivity from the daughter product(s) is a desirable feature for
radiopharmaceuticals.
![{\displaystyle {\ce {^{99\!m}_{43}Tc->[{\ce {\gamma \ 141keV}}][{\ce {6h}}]{}_{43}^{99}Tc->[{\ce {\beta ^{-}\ 249keV}}][211,000\ {\ce {y}}]\overbrace {\underset {(stable)}{^{99}_{44}Ru}} ^{ruthenium-99}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8216cabc609fe3a81bc58bb85c664bd852c4c736)
Production
Production of Mo-99 in nuclear reactors
- Neutron irradiation of U-235 targets
Nuclear reactors producing 99Mo from U-235 targets. The year indicates the date of the first criticality of the reactor.
| Type
|
Reactor
|
Location
|
Target/Fuel
|
Year
|
| Large-scale producers |
NRU |
Canada |
HEU/LEU |
1957
|
| BR2 |
Belgium |
HEU/HEU |
1961
|
| SAFARI-1 |
South Africa |
LEU/LEU |
1965
|
| HFR |
the Netherlands |
HEU/LEU |
1961
|
| Osiris reactor |
France |
LEU/HEU |
1966
|
| Regional producers |
OPAL |
Australia |
LEU/LEU |
2006
|
| MPR RSG-GAS |
Indonesia |
LEU/LEU |
1987
|
| RA-3 |
Argentina |
LEU/LEU |
1961
|
| MARIA |
Poland |
HEU/HEU |
1974
|
| LVR-15 |
Czech Republic |
HEU/HEU |
1957
|
- Neutron activation of Mo-98
Production of
99Mo by
neutron activation of natural molybdenum, or molybdenum enriched in Mo-98, is another, currently smaller, route of production.
Production of Tc-99m/Mo-99 in particle accelerators
- Production of "Instant" Tc-99m
- Indirect routes of production of Mo-99
Other particle accelerator-based isotope production techniques have
been investigated. The supply disruptions of Mo-99 in the late 2000s and
the aging of the producing nuclear reactors forced the industry to look
into alternative methods of production. The use of cyclotrons to
produce Mo-99 from Mo-100 via (n,2n) or (γ,n) reactions has been further
investigated.
Technetium-99m generators
Technetium-99m's short half-life of 6 hours makes storage impossible
and would make transport very expensive. It is instead its parent
nuclide
99Mo is supplied to hospitals after its extraction
from the neutron-irradiated uranium targets and its purification in
dedicated processing facilities. It is shipped by specialised radiopharmaceutical companies in the form of
technetium-99m generators
worldwide or directly distributed to the local market. The generators,
colloquially known as a moly cows, are devices designed to provide
radiation shielding for transport and to minimize the extraction work
done at the medical facility. A typical dose rate at 1 metre from Tc-99m
generator is 20-50
μSv/h during transport. These generators' output declines with time and must be replaced weekly, since the half-life of
99Mo is still only 66 hours.
Molybdenum-99 spontaneously decays to excited states of
99Tc through
beta decay. Over 87% of the decays lead to the 142 keV excited state of Tc-99m. A
β− electron and a
ν
e electron antineutrino are emitted in the process (
99Mo →
99mTc +
β− +
ν
e). The
β− electrons are easily
shielded for transport, and
99mTc generators are only minor radiation hazards, mostly due to secondary X-rays produced by the electrons (also known as
Bremsstrahlung).
At the hospital, the
99mTc that forms through
99Mo decay is chemically extracted from the technetium-99m generator. Most commercial
99Mo/
99mTc generators use
column chromatography, in which
99Mo in the form of water-soluble molybdate, MoO
42− is
adsorbed onto acid alumina (Al
2O
3). When the
99Mo decays, it forms
pertechnetate TcO
4−,
which, because of its single charge, is less tightly bound to the
alumina. Pulling normal saline solution through the column of
immobilized
99MoO
42− elutes the soluble
99mTcO
4−, resulting in a saline solution containing the
99mTc as the dissolved
sodium salt of the pertechnetate. One technetium-99m generator, holding only a few micrograms of
99Mo, can potentially diagnose 10,000 patients because it will be producing
99mTc strongly for over a week.
Preparation
Technetium exits the generator in the form of the pertechnetate ion, TcO
4−. The
oxidation state of Tc in this compound is +7. This is directly suitable for medical applications only in
bone scans
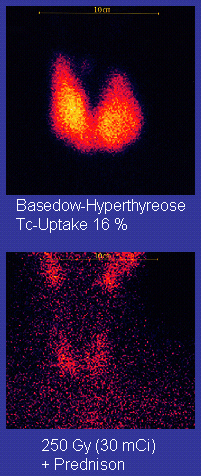
(it is taken up by osteoblasts) and some thyroid scans (it is taken up
in place of iodine by normal thyroid tissues). In other types of scans
relying on Tc-99m, a
reducing agent is added to the pertechnetate solution to bring the oxidation state of the Tc down to +3 or +4. Secondly, a
ligand is added to form a
coordination complex. The ligand is chosen to have an affinity for the specific organ to be targeted. For example, the
exametazime
complex of Tc in oxidation state +3 is able to cross the blood–brain
barrier and flow through the vessels in the brain for cerebral blood
flow imaging. Other ligands include
sestamibi for myocardial perfusion imaging and mercapto acetyl triglycine for
MAG3 scan to measure renal function.
Medical uses
In
1970, Eckelman and Richards presented the first "kit" containing all
the ingredients required to release the Tc-99m, "milked" from the
generator, in the chemical form to be administered to the patient.
Technetium-99m is used in 20 million diagnostic
nuclear medical procedures every year. Approximately 85% of diagnostic imaging procedures in nuclear medicine use this isotope as
radioactive tracer. Klaus Schwochau's book
Technetium lists 31
radiopharmaceuticals based on
99mTc for imaging and functional studies of the
brain,
myocardium,
thyroid,
lungs,
liver,
gallbladder,
kidneys,
skeleton,
blood, and
tumors. Depending on the procedure, the
99mTc is tagged (or bound to) a pharmaceutical that transports it to its required location. For example, when
99mTc is chemically bound to
exametazime
(HMPAO), the drug is able to cross the blood–brain barrier and flow
through the vessels in the brain for cerebral blood-flow imaging. This
combination is also used for labeling white blood cells
(99mTc labeled WBC) to visualize sites of infection.
99mTc sestamibi is used for myocardial perfusion imaging, which shows how well the blood flows through the heart. Imaging to measure
renal function is done by attaching
99mTc to mercaptoacetyl triglycine (
MAG3); this procedure is known as a
MAG3 scan.
Technetium-99m can be readily detected in the body by medical equipment because it emits 140.5
keV gamma rays (these are about the same wavelength as emitted by conventional X-ray diagnostic equipment), and its
half-life for gamma emission is six hours (meaning 94% of it decays to
99Tc in 24 hours). The "short" physical
half-life of the isotope and its
biological half-life
of 1 day (in terms of human activity and metabolism) allows for
scanning procedures which collect data rapidly, but keep total patient
radiation exposure low.
Radiation side-effects
Diagnostic
treatment involving technetium-99m will result in radiation exposure to
technicians, patients, and passers-by. Typical quantities of technetium
administered for immunoscintigraphy tests, such as
SPECT tests, range from 400 to 1,100 MBq (11 to 30 mCi) (
millicurie or mCi; and Mega-
Becquerel or MBq) for adults. These doses result in radiation exposures to the patient around 10 m
Sv (1000
mrem), the equivalent of about 500
chest X-ray exposures. This level of radiation exposure carries a 1 in 1000 lifetime risk of developing a solid cancer or leukemia in the patient. The risk is higher in younger patients, and lower in older ones.
Unlike a chest x-ray, the radiation source is inside the patient and
will be carried around for a few days, exposing others to second-hand
radiation. A spouse who stays constantly by the side of the patient
through this time might receive one thousandth of patient's radiation
dose this way.
The short half-life of the isotope allows for scanning procedures
that collect data rapidly. The isotope is also of a very low energy
level for a gamma emitter. Its ~140 keV of energy make it safer for use
because of the substantially reduced
ionization compared with other gamma emitters. The energy of gammas from
99mTc
is about the same as the radiation from a commercial diagnostic X-ray
machine, although the number of gammas emitted results in radiation
doses more comparable to X-ray studies like
computed tomography.
Technetium-99m has several features that make it safer than other
possible isotopes. Its gamma decay mode can be easily detected by a
camera, allowing the use of smaller quantities. And because
technetium-99m has a short half-life, its quick decay into the far less
radioactive technetium-99 results in relatively low total radiation dose
to the patient per unit of initial activity after administration, as
compared to other radioisotopes. In the form administered in these
medical tests (usually pertechnetate), technetium-99m and technetium-99
are eliminated from the body within a few days.
3-D scanning technique: SPECT
Single photon emission computed tomography (SPECT) is a
nuclear medicine imaging technique
using gamma rays. It may be used with any gamma-emitting isotope,
including Tc-99m. In the use of technetium-99m, the radioisotope is
administered to the patient and the escaping gamma rays are incident
upon a moving
gamma camera
which computes and processes the image. To acquire SPECT images, the
gamma camera is rotated around the patient. Projections are acquired at
defined points during the rotation, typically every three to six
degrees. In most cases, a full 360° rotation is used to obtain an
optimal reconstruction. The time taken to obtain each projection is also
variable, but 15–20 seconds are typical. This gives a total scan time
of 15–20 minutes.
The technetium-99m radioisotope is used predominantly in bone and brain scans. For
bone scans,
the pertechnetate ion is used directly, as it is taken up by
osteoblasts attempting to heal a skeletal injury, or (in some cases) as a
reaction of these cells to a tumor (either primary or metastatic) in
the bone. In brain scanning, Tc-99m is attached to the chelating agent
HMPAO to create
technetium (99mTc) exametazime,
an agent which localizes in the brain according to region blood flow,
making it useful for the detection of stroke and dementing illnesses
that decrease regional brain flow and metabolism.
Most recently, technetium-99m scintigraphy has been combined with CT coregistration technology to produce
SPECT/CT
scans. These employ the same radioligands and have the same uses as
SPECT scanning, but are able to provide even finer 3-D localization of
high-uptake tissues, in cases where finer resolution is needed. An
example is the
sestamibi parathyroid scan which is performed using the Tc-99m radioligand
sestamibi, and can be done in either SPECT or SPECT/CT machines.
Bone scan
The
nuclear medicine technique commonly called the
bone scan usually uses Tc-99m. It is not to be confused with the "bone density scan",
DEXA,
which is a low-exposure X-ray test measuring bone density to look for
osteoporosis and other diseases where bones lose mass without rebuilding
activity. The nuclear medicine technique is sensitive to areas of
unusual bone rebuilding activity, since the radiopharmaceutical is taken
up by
osteoblast
cells which build bone. The technique therefore is sensitive to
fractures and bone reaction to bone tumors, including metastases. For a
bone scan, the patient is injected with a small amount of radioactive
material, such as 700–1,100 MBq (19–30 mCi) of
99mTc-medronic acid and then scanned with a
gamma camera. Medronic acid is a
phosphate
derivative which can exchange places with bone phosphate in regions of
active bone growth, so anchoring the radioisotope to that specific
region. To view small lesions (less than 1 centimetre (0.39 in))
especially in the spine, the
SPECT
imaging technique may be required, but currently in the United States,
most insurance companies require separate authorization for SPECT
imaging.
Myocardial perfusion imaging
Myocardial perfusion imaging (MPI) is a form of functional cardiac imaging, used for the diagnosis of
ischemic heart disease. The underlying principle is, under conditions of stress, diseased
myocardium receives less blood flow than normal myocardium. MPI is one of several types of
cardiac stress test. As a
nuclear stress test
the average radiation exposure is 9.4 mSV which compared to a typical 2
view Chest X-Ray (.1 mSV) is equivalent to 94 Chest X-Rays.
Several radiopharmaceuticals and radionuclides may be used for
this, each giving different information. In the myocardial perfusion
scans using Tc-99m, the radiopharmaceuticals
99mTc-
tetrofosmin (Myoview,
GE Healthcare) or
99mTc-
sestamibi (Cardiolite,
Bristol-Myers Squibb) are used. Following this, myocardial stress is induced, either by exercise or pharmacologically with
adenosine,
dobutamine or
dipyridamole(Persantine), which increase the heart rate or by
regadenoson(Lexiscan), a vasodilator. (
Aminophylline
can be used to reverse the effects of dipyridamole and regadenoson).
Scanning may then be performed with a conventional gamma camera, or with
SPECT/CT.
Cardiac ventriculography
Functional brain imaging
Usually the gamma-emitting tracer used in functional brain imaging is
99mTc-HMPAO (hexamethylpropylene amine oxime,
exametazime). The similar
99mTc-EC
tracer may also be used. These molecules are preferentially distributed
to regions of high brain blood flow, and act to assess brain metabolism
regionally, in an attempt to diagnose and differentiate the different
causal pathologies of
dementia. When used with the 3-D
SPECT technique, they compete with brain
FDG-PET scans and
fMRI brain scans as techniques to map the regional metabolic rate of brain tissue.
Sentinel-node identification
The radioactive properties of
99mTc can be used to identify the predominant
lymph nodes draining a cancer, such as
breast cancer or
malignant melanoma. This is usually performed at the time of
biopsy or
resection.
99mTc-labelled
isosulfan blue dye
is injected intradermally around the intended biopsy site. The general
location of the sentinel node is determined with the use of a handheld
scanner with a gamma-sensor probe that detects the
technetium-99m–labeled sulfur colloid that was previously injected
around the biopsy site. An incision is then made over the area of
highest radionuclide accumulation, and the sentinel node is identified
within the incision by inspection; the isosulfan blue dye will usually
stain any draining nodes blue.
Immunoscintigraphy
Immunoscintigraphy incorporates
99mTc into a
monoclonal antibody, an
immune system protein, capable of binding to
cancer cells. A few hours after injection, medical equipment is used to detect the gamma rays emitted by the
99mTc;
higher concentrations indicate where the tumor is. This technique is
particularly useful for detecting hard-to-find cancers, such as those
affecting the
intestines. These modified antibodies are sold by the German company
Hoechst (now part of
Sanofi-Aventis) under the name "Scintium".
Blood pool labeling
Pyrophosphate for heart damage
Sulfur colloid for spleen scan
The
sulfur colloid of
99mTc is scavenged by the
spleen, making it possible to image the structure of the spleen.
Meckel's diverticulum
Pertechnetate is actively accumulated and secreted by the mucoid cells of the gastric mucosa,
and therefore, technetate(VII) radiolabeled with Tc99m is injected into
the body when looking for ectopic gastric tissue as is found in a
Meckel's diverticulum with Meckel's Scans.





![{\displaystyle {\ce {^{99\!m}_{43}Tc->[{\ce {\gamma \ 141keV}}][{\ce {6h}}]{}_{43}^{99}Tc->[{\ce {\beta ^{-}\ 249keV}}][211,000\ {\ce {y}}]\overbrace {\underset {(stable)}{^{99}_{44}Ru}} ^{ruthenium-99}}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/8216cabc609fe3a81bc58bb85c664bd852c4c736)