From Wikipedia, the free encyclopedia
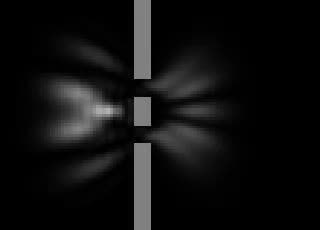
Photons or particles of matter (like an electron) produce a wave pattern when two slits are used
Light from a green laser passing through two slits 0.4mm wide and 0.1mm apart
In modern physics, the double-slit experiment
is a demonstration that light and matter can display characteristics of
both classically defined waves and particles; moreover, it displays the
fundamentally probabilistic nature of quantum mechanical phenomena. This type of experiment was first performed, using light, by Thomas Young in 1801, as a demonstration of the wave behavior of light. At that time it was thought that light consisted of either waves or
particles. With the beginning of modern physics, about a hundred years
later, it was realized that light could in fact show behavior
characteristic of both waves and particles. In 1927, Davisson and Germer demonstrated that electrons show the same behavior, which was later extended to atoms and molecules. Thomas Young's experiment with light was part of classical physics well before quantum mechanics, and the concept of wave-particle duality. He believed it demonstrated that the wave theory of light was correct, and his experiment is sometimes referred to as Young's experiment or Young's slits.
The experiment belongs to a general class of "double path" experiments,
in which a wave is split into two separate waves that later combine into
a single wave. Changes in the path-lengths of both waves result in a phase shift, creating an interference pattern. Another version is the Mach–Zehnder interferometer, which splits the beam with a beam splitter.
In the basic version of this experiment, a coherent light source, such as a laser
beam, illuminates a plate pierced by two parallel slits, and the light
passing through the slits is observed on a screen behind the plate. The wave nature of light causes the light waves passing through the two slits to interfere, producing bright and dark bands on the screen – a result that would not be expected if light consisted of classical particles.
However, the light is always found to be absorbed at the screen at
discrete points, as individual particles (not waves); the interference
pattern appears via the varying density of these particle hits on the
screen. Furthermore, versions of the experiment that include detectors at the slits find that each detected photon passes through one slit (as would a classical particle), and not through both slits (as would a wave). However, such experiments
demonstrate that particles do not form the interference pattern if one
detects which slit they pass through. These results demonstrate the
principle of wave–particle duality.
Other atomic-scale entities, such as electrons, are found to exhibit the same behavior when fired towards a double slit.
Additionally, the detection of individual discrete impacts is observed
to be inherently probabilistic, which is inexplicable using classical mechanics.
The experiment can be done with entities much larger than
electrons and photons, although it becomes more difficult as size
increases. The largest entities for which the double-slit experiment has
been performed were molecules that each comprised 810 atoms (whose total mass was over 10,000 atomic mass units).
The double-slit experiment (and its variations) has become a
classic for its clarity in expressing the central puzzles of quantum
mechanics. Because it demonstrates the fundamental limitation of the
ability of the observer to predict experimental results, Richard Feynman called it "a phenomenon which is impossible […] to explain in any classical way, and which has in it the heart of quantum mechanics. In reality, it contains the only mystery [of quantum mechanics]."
Numerical
simulation of the double-slit experiment with electrons. Figure on the
left: evolution (from left to right) of the intensity of the electron
beam at the exit of the slits (left) up to the detection screen located
10cm after the slits (right). The higher the intensity, the more the
color is light blue - Figure in the center: impacts of the electrons
observed on the screen - Figure on the right: intensity of the electrons
in the
far field
approximation (on the screen). Numerical data from Claus Jönsson's
experiment (1961). Photons, atoms and molecules follow a similar
evolution.
Overview
Same double-slit assembly (0.7 mm between slits); in top image, one slit is closed. In the single-slit image, a
diffraction pattern
(the faint spots on either side of the main band) forms due to the
nonzero width of the slit. This diffraction pattern is also seen in the
double-slit image, but with many smaller interference fringes.
If light consisted strictly of ordinary or classical
particles, and these particles were fired in a straight line through a
slit and allowed to strike a screen on the other side, we would expect
to see a pattern corresponding to the size and shape of the slit.
However, when this "single-slit experiment" is actually performed, the
pattern on the screen is a diffraction pattern
in which the light is spread out. The smaller the slit, the greater the
angle of spread. The top portion of the image shows the central portion
of the pattern formed when a red laser illuminates a slit and, if one
looks carefully, two faint side bands. More bands can be seen with a
more highly refined apparatus. Diffraction explains the pattern as being the result of the interference of light waves from the slit.
Simulation
of a particle wave function: double slit experiment. The white blur
represents the wave. The whiter the pixel, the greater the probability
of finding a particle in that place if measured.
If one illuminates two parallel slits, the light from the two slits
again interferes. Here the interference is a more pronounced pattern
with a series of alternating light and dark bands. The width of the
bands is a property of the frequency of the illuminating light. (See the bottom photograph to the right.) When Thomas Young
(1773–1829) first demonstrated this phenomenon, it indicated that light
consists of waves, as the distribution of brightness can be explained
by the alternately additive and subtractive interference of wavefronts.
Young's experiment, performed in the early 1800s, played a crucial role
in the understanding of the wave theory of light, vanquishing the corpuscular theory of light proposed by Isaac Newton, which had been the accepted model of light propagation in the 17th and 18th centuries. However, the later discovery of the photoelectric effect
demonstrated that under different circumstances, light can behave as if
it is composed of discrete particles. These seemingly contradictory
discoveries made it necessary to go beyond classical physics and take
the quantum nature of light into account.
Feynman was fond of saying that all of quantum mechanics can be
gleaned from carefully thinking through the implications of this single
experiment.
He also proposed (as a thought experiment) that if detectors were
placed before each slit, the interference pattern would disappear.
The Englert–Greenberger duality relation provides a detailed treatment of the mathematics of double-slit interference in the context of quantum mechanics.
A low-intensity double-slit experiment was first performed by G. I. Taylor in 1909, by reducing the level of incident light until photon emission/absorption events were mostly non-overlapping.
A double-slit experiment was not performed with anything other than light until 1961, when Claus Jönsson of the University of Tübingen performed it with electron beams. In 1974, the Italian physicists Pier Giorgio Merli, Gian Franco Missiroli, and Giulio Pozzi
repeated the experiment using single electrons and biprism (instead of
slits), showing that each electron interferes with itself as predicted
by quantum theory. In 2002, the single-electron version of the experiment was voted "the most beautiful experiment" by readers of Physics World.
In 2012, Stefano Frabboni and co-workers eventually performed the
double-slit experiment with electrons and real slits, following the
original scheme proposed by Feynman. They sent single electrons onto
nanofabricated slits (about 100 nm wide) and, by collecting the
transmitted electrons with a single-electron detector, they could show
the build-up of a double-slit interference pattern.
In 2019, single particle interference was demonstrated for antimatter by Marco Giammarchi and coworkers.
Variations of the experiment
Interference of individual particles
Buildup of interference pattern from individual particle detections
An important version of this experiment involves single particles.
Sending particles through a double-slit apparatus one at a time results
in single particles appearing on the screen, as expected. Remarkably,
however, an interference pattern emerges when these particles are
allowed to build up one by one (see the adjacent image). This
demonstrates the wave–particle duality,
which states that all matter exhibits both wave and particle
properties: the particle is measured as a single pulse at a single
position, while the wave describes the probability of absorbing the particle at a specific place on the screen. This phenomenon has been shown to occur with photons, electrons, atoms and even some molecules, including buckyballs.
The probability of detection is the square of the amplitude of the wave and can be calculated with classical waves (see below).
Ever since the origination of quantum mechanics, some theorists have
searched for ways to incorporate additional determinants or "hidden variables" that, were they to become known, would account for the location of each individual impact with the target.
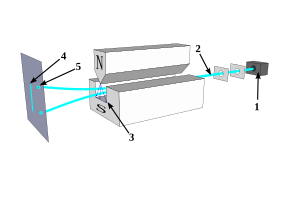
Mach-Zehnder interferometer
The Mach–Zehnder interferometer can be seen as a simplified version
of the double-slit experiment. Instead of propagating through free space
after the two slits, and hitting any position in an extended screen, in
the interferometer the photons can only propagate via two paths, and
hit two discrete photodetectors. This makes it possible to describe it
via simple linear algebra in dimension 2, rather than differential
equations.
A photon emitted by the laser hits the first beam splitter and is
then in a superposition between the two possible paths. In the second
beam splitter these paths interfere, causing the photon to hit the
photodetector on the right with probability one, and the photodetector
on the bottom with probability zero. It is interesting to consider what
would happen if the photon were definitely in either of paths between
the beam splitters. This can be accomplished by blocking one of the
paths, or equivalently by detecting the presence of a photon there. In
both cases there will be no interference between the paths anymore, and
both photodectors will be hit with probability 1/2. From this we can
conclude that the photon does not take one path or another after the
first beam splitter, but rather that it is in a genuine quantum
superposition of the two paths.
"Which-way" experiments and the principle of complementarity
A well-known thought experiment
predicts that if particle detectors are positioned at the slits,
showing through which slit a photon goes, the interference pattern will
disappear. This which-way experiment illustrates the complementarity principle that photons can behave as either particles or waves, but cannot be observed as both at the same time.
Despite the importance of this thought experiment in the history of quantum mechanics (for example, see the discussion on Einstein's version of this experiment), technically feasible realizations of this experiment were not proposed until the 1970s. (Naive implementations of the textbook gedanken
experiment are not possible because photons cannot be detected without
absorbing the photon.) Currently, multiple experiments have been
performed illustrating various aspects of complementarity.
An experiment performed in 1987
produced results that demonstrated that information could be obtained
regarding which path a particle had taken without destroying the
interference altogether. This showed the effect of measurements that
disturbed the particles in transit to a lesser degree and thereby
influenced the interference pattern only to a comparable extent. In
other words, if one does not insist that the method used to determine
which slit each photon passes through be completely reliable, one can
still detect a (degraded) interference pattern.
Delayed choice and quantum eraser variations
A
diagram of Wheeler's delayed choice experiment, showing the principle
of determining the path of the photon after it passes through the slit
Wheeler's delayed choice experiments
demonstrate that extracting "which path" information after a particle
passes through the slits can seem to retroactively alter its previous
behavior at the slits.
Quantum eraser
experiments demonstrate that wave behavior can be restored by erasing
or otherwise making permanently unavailable the "which path"
information.
A simple do-it-at-home illustration of the quantum eraser phenomenon was given in an article in Scientific American.
If one sets polarizers before each slit with their axes orthogonal to
each other, the interference pattern will be eliminated. The polarizers
can be considered as introducing which-path information to each beam.
Introducing a third polarizer in front of the detector with an axis of
45° relative to the other polarizers "erases" this information, allowing
the interference pattern to reappear. This can also be accounted for by
considering the light to be a classical wave, and also when using circular polarizers and single photons. Implementations of the polarizers using entangled photon pairs have no classical explanation.
Weak measurement
In a highly publicized experiment in 2012, researchers claimed to
have identified the path each particle had taken without any adverse
effects at all on the interference pattern generated by the particles.
In order to do this, they used a setup such that particles coming to
the screen were not from a point-like source, but from a source with two
intensity maxima. However, commentators such as Svensson have pointed out that there is in fact no conflict between the weak measurements performed in this variant of the double-slit experiment and the Heisenberg uncertainty principle.
Weak measurement followed by post-selection did not allow simultaneous
position and momentum measurements for each individual particle, but
rather allowed measurement of the average trajectory of the particles
that arrived at different positions. In other words, the experimenters
were creating a statistical map of the full trajectory landscape.
Other variations
A laboratory double-slit assembly; distance between top posts approximately 2.5 cm (one inch).
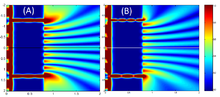
Near-field intensity distribution patterns for plasmonic slits with equal widths (A) and non-equal widths (B).
In 1967, Pfleegor and Mandel demonstrated two-source interference using two separate lasers as light sources.
It was shown experimentally in 1972 that in a double-slit system
where only one slit was open at any time, interference was nonetheless
observed provided the path difference was such that the detected photon
could have come from either slit. The experimental conditions were such that the photon density in the system was much less than unity.
In 1999, the double-slit experiment was successfully performed
with buckyball molecules (each of which comprises 60 carbon atoms). A buckyball is large enough (diameter about 0.7 nm, nearly half a million times larger than a proton) to be seen under an electron microscope.
In 2005, E. R. Eliel presented an experimental and theoretical
study of the optical transmission of a thin metal screen perforated by
two subwavelength slits, separated by many optical wavelengths. The
total intensity of the far-field double-slit pattern is shown to be
reduced or enhanced as a function of the wavelength of the incident
light beam.
In 2012, researchers at the University of Nebraska–Lincoln performed the double-slit experiment with electrons as described by Richard Feynman,
using new instruments that allowed control of the transmission of the
two slits and the monitoring of single-electron detection events.
Electrons were fired by an electron gun and passed through one or two
slits of 62 nm wide × 4 μm tall.
In 2013, the double-slit experiment was successfully performed
with molecules that each comprised 810 atoms (whose total mass was over
10,000 atomic mass units). The record was raised to 2000 atoms (25,000 amu) in 2019.
Hydrodynamic pilot wave analogs
Hydrodynamic analogs
have been developed that can recreate various aspects of quantum
mechanical systems, including single-particle interference through a
double-slit.
A silicone oil droplet, bouncing along the surface of a liquid,
self-propels via resonant interactions with its own wave field. The
droplet gently sloshes the liquid with every bounce. At the same time,
ripples from past bounces affect its course. The droplet's interaction
with its own ripples, which form what is known as a pilot wave,
causes it to exhibit behaviors previously thought to be peculiar to
elementary particles – including behaviors customarily taken as evidence
that elementary particles are spread through space like waves, without
any specific location, until they are measured.
Behaviors mimicked via this hydrodynamic pilot-wave system include quantum single particle diffraction,
tunneling, quantized orbits, orbital level splitting, spin, and
multimodal statistics. It is also possible to infer uncertainty
relations and exclusion principles. Videos are available illustrating
various features of this system.
However, more complicated systems that involve two or more
particles in superposition are not amenable to such a simple,
classically intuitive explanation. Accordingly, no hydrodynamic analog of entanglement has been developed. Nevertheless, optical analogs are possible.
Classical wave-optics formulation
Two-slit diffraction pattern by a plane wave
Photo of the double-slit interference of sunlight.
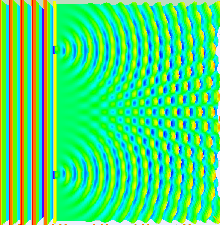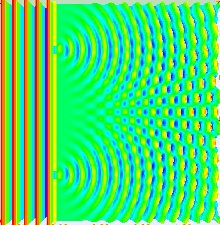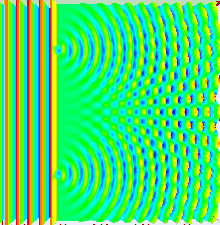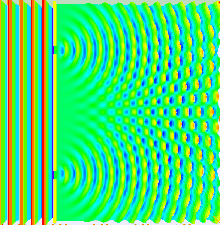
Two slits are illuminated by a plane wave.
Much of the behaviour of light can be modelled using classical wave theory. The Huygens–Fresnel principle
is one such model; it states that each point on a wavefront generates a
secondary wavelet, and that the disturbance at any subsequent point can
be found by summing the contributions of the individual wavelets at that point. This summation needs to take into account the phase as well as the amplitude of the individual wavelets. Only the intensity of a light field can be measured—this is proportional to the square of the amplitude.
In the double-slit experiment, the two slits are illuminated by a
single laser beam. If the width of the slits is small enough (less
than the wavelength of the laser light), the slits diffract the light
into cylindrical waves. These two cylindrical wavefronts are
superimposed, and the amplitude, and therefore the intensity, at any
point in the combined wavefronts depends on both the magnitude and the
phase of the two wavefronts. The difference in phase between the two
waves is determined by the difference in the distance travelled by the
two waves.
If the viewing distance is large compared with the separation of the slits (the far field),
the phase difference can be found using the geometry shown in the
figure below right. The path difference between two waves travelling at
an angle θ is given by:

Where d is the distance between the two slits. When the two waves are
in phase, i.e. the path difference is equal to an integral number of
wavelengths, the summed amplitude, and therefore the summed intensity is
maximum, and when they are in anti-phase, i.e. the path difference is
equal to half a wavelength, one and a half wavelengths, etc., then the
two waves cancel and the summed intensity is zero. This effect is known
as interference. The interference fringe maxima occur at angles

where λ is the wavelength of the light. The angular spacing of the fringes, θf, is given by

The spacing of the fringes at a distance z from the slits is given by

For example, if two slits are separated by 0.5 mm (d), and are illuminated with a 0.6μm wavelength laser (λ), then at a distance of 1m (z), the spacing of the fringes will be 1.2 mm.
If the width of the slits b is greater than the wavelength, the Fraunhofer diffraction equation gives the intensity of the diffracted light as:
![{\begin{aligned}I(\theta )&\propto \cos ^{2}\left[{\frac {\pi d\sin \theta }{\lambda }}\right]~\mathrm {sinc} ^{2}\left[{\frac {\pi b\sin \theta }{\lambda }}\right]\end{aligned}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5fb832693cac4bc3093639a4c6ccf7916e76a06a)
Where the sinc function is defined as sinc(x) = sin(x)/x for x ≠ 0, and sinc(0) = 1.
This is illustrated in the figure above, where the first pattern is the diffraction pattern of a single slit, given by the sinc
function in this equation, and the second figure shows the combined
intensity of the light diffracted from the two slits, where the cos
function represent the fine structure, and the coarser structure
represents diffraction by the individual slits as described by the sinc function.
Similar calculations for the near field can be done using the Fresnel diffraction
equation. As the plane of observation gets closer to the plane in which
the slits are located, the diffraction patterns associated with each
slit decrease in size, so that the area in which interference occurs is
reduced, and may vanish altogether when there is no overlap in the two
diffracted patterns.
Interpretations of the experiment
Like the Schrödinger's cat thought experiment, the double-slit experiment is often used to highlight the differences and similarities between the various interpretations of quantum mechanics.
Copenhagen interpretation
The Copenhagen interpretation,
put forth by some of the pioneers in the field of quantum mechanics,
asserts that it is undesirable to posit anything that goes beyond the
mathematical formulae and the kinds of physical apparatus and reactions
that enable us to gain some knowledge of what goes on at the atomic
scale. One of the mathematical constructs that enables experimenters to
predict very accurately certain experimental results is sometimes called
a probability wave. In its mathematical form it is analogous to the
description of a physical wave, but its "crests" and "troughs" indicate
levels of probability for the occurrence of certain phenomena (e.g., a
spark of light at a certain point on a detector screen) that can be
observed in the macro world of ordinary human experience.
The probability "wave" can be said to "pass through space"
because the probability values that one can compute from its
mathematical representation are dependent on time. One cannot speak of
the location of any particle such as a photon between the time it is
emitted and the time it is detected simply because in order to say that
something is located somewhere at a certain time one has to detect it.
The requirement for the eventual appearance of an interference pattern
is that particles be emitted, and that there be a screen with at least
two distinct paths for the particle to take from the emitter to the
detection screen. Experiments observe nothing whatsoever between the
time of emission of the particle and its arrival at the detection
screen. If a ray tracing is next made as if a light wave (as understood
in classical physics) is wide enough to take both paths, then that ray
tracing will accurately predict the appearance of maxima and minima on
the detector screen when many particles pass through the apparatus and
gradually "paint" the expected interference pattern.
Path-integral formulation
One of an infinite number of equally likely paths used in the Feynman path integral
The Copenhagen interpretation is similar to the path integral formulation
of quantum mechanics provided by Feynman. The path integral
formulation replaces the classical notion of a single, unique trajectory
for a system, with a sum over all possible trajectories. The
trajectories are added together by using functional integration.
Each path is considered equally likely, and thus contributes the same amount. However, the phase of this contribution at any given point along the path is determined by the action along the path:

All these contributions are then added together, and the magnitude of the final result is squared, to get the probability distribution for the position of a particle:

As is always the case when calculating probability, the results must then be normalized by imposing:

To summarize, the probability distribution of the outcome is the normalized square of the norm of the superposition, over all paths from the point of origin to the final point, of waves propagating proportionally
to the action along each path. The differences in the cumulative
action along the different paths (and thus the relative phases of the
contributions) produces the interference pattern
observed by the double-slit experiment. Feynman stressed that his
formulation is merely a mathematical description, not an attempt to
describe a real process that we can measure.
Relational interpretation
An
example of the uncertainty principle related to the relational
interpretation. The more that is known about the position of a particle,
the less is known about the velocity, and vice versa
According to the relational interpretation of quantum mechanics, first proposed by Carlo Rovelli, observations such as those in the double-slit experiment result specifically from the interaction between the observer
(measuring device) and the object being observed (physically interacted
with), not any absolute property possessed by the object. In the case
of an electron, if it is initially "observed" at a particular slit, then
the observer–particle (photon–electron) interaction includes
information about the electron's position. This partially constrains the
particle's eventual location at the screen. If it is "observed"
(measured with a photon) not at a particular slit but rather at the
screen, then there is no "which path" information as part of the
interaction, so the electron's "observed" position on the screen is
determined strictly by its probability function. This makes the
resulting pattern on the screen the same as if each individual electron
had passed through both slits.
Many-worlds interpretation
Physicist David Deutsch argues in his book The Fabric of Reality that the double-slit experiment is evidence for the many-worlds interpretation.
However, since every interpretation of quantum mechanics is empirically
indistinguishable, some scientists are skeptical of this claim.
De Broglie–Bohm theory
Trajectories of particles under De Broglie–Bohm theory in the double-slit experiment.
An alternative to the standard understanding of quantum mechanics, the De Broglie–Bohm theory
states that particles also have precise locations at all times, and
that their velocities are defined by the wave-function. So while a
single particle will travel through one particular slit in the
double-slit experiment, the so-called "pilot wave" that influences it
will travel through both. The two slit de Broglie-Bohm trajectories were
first calculated by Chris Dewdney while working with Chris Philippidis
and Basil Hiley at Birkbeck College (London).
The de Broglie-Bohm theory produces the same statistical results as
standard quantum mechanics, but dispenses with many of its conceptual
difficulties.
100
trajectories guided by the wave function. In De Broglie-Bohm's theory, a
particle is represented, at any time, by a wave function
and a position (center of mass). This is a kind of augmented reality compared to the standard interpretation.
































![{\begin{aligned}I(\theta )&\propto \cos ^{2}\left[{\frac {\pi d\sin \theta }{\lambda }}\right]~\mathrm {sinc} ^{2}\left[{\frac {\pi b\sin \theta }{\lambda }}\right]\end{aligned}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5fb832693cac4bc3093639a4c6ccf7916e76a06a)







