| General properties | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pronunciation | /ˈsɪlɪkən/ |
||||||||||||||||||||||||||||||
| Appearance | crystalline, reflective with bluish-tinged faces | ||||||||||||||||||||||||||||||
| Standard atomic weight (Ar, standard) | [28.084, 28.086] conventional: 28.085 | ||||||||||||||||||||||||||||||
| Silicon in the periodic table | |||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
| Atomic number (Z) | 14 | ||||||||||||||||||||||||||||||
| Group | group 14 (carbon group) | ||||||||||||||||||||||||||||||
| Period | period 3 | ||||||||||||||||||||||||||||||
| Element category | metalloid | ||||||||||||||||||||||||||||||
| Block | p-block | ||||||||||||||||||||||||||||||
| Electron configuration | [Ne] 3s2 3p2 | ||||||||||||||||||||||||||||||
Electrons per shell
|
2, 8, 4 | ||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||
| Melting point | 1687 K (1414 °C, 2577 °F) | ||||||||||||||||||||||||||||||
| Boiling point | 3538 K (3265 °C, 5909 °F) | ||||||||||||||||||||||||||||||
| Density (near r.t.) | 2.3290 g/cm3 | ||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 2.57 g/cm3 | ||||||||||||||||||||||||||||||
| Heat of fusion | 50.21 kJ/mol | ||||||||||||||||||||||||||||||
| Heat of vaporization | 383 kJ/mol | ||||||||||||||||||||||||||||||
| Molar heat capacity | 19.789 J/(mol·K) | ||||||||||||||||||||||||||||||
Vapor pressure
|
|||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||
| Oxidation states | 4, 3, 2, 1[1] −1, −2, −3, −4 |
||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.90 | ||||||||||||||||||||||||||||||
| Ionization energies |
|
||||||||||||||||||||||||||||||
| Atomic radius | empirical: 111 pm | ||||||||||||||||||||||||||||||
| Covalent radius | 111 pm | ||||||||||||||||||||||||||||||
| Van der Waals radius | 210 pm | ||||||||||||||||||||||||||||||
| Miscellanea | |||||||||||||||||||||||||||||||
| Crystal structure | face-centered diamond-cubic
|
||||||||||||||||||||||||||||||
| Speed of sound thin rod | 8433 m/s (at 20 °C) | ||||||||||||||||||||||||||||||
| Thermal expansion | 2.6 µm/(m·K) (at 25 °C) | ||||||||||||||||||||||||||||||
| Thermal conductivity | 149 W/(m·K) | ||||||||||||||||||||||||||||||
| Electrical resistivity | 2.3×103 Ω·m (at 20 °C)[2] | ||||||||||||||||||||||||||||||
| Band gap | 1.12 eV (at 300 K) | ||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[3] | ||||||||||||||||||||||||||||||
| Magnetic susceptibility | −3.9·10−6 cm3/mol (298 K)[4] | ||||||||||||||||||||||||||||||
| Young's modulus | 130–188 GPa[5] | ||||||||||||||||||||||||||||||
| Shear modulus | 51–80 GPa[5] | ||||||||||||||||||||||||||||||
| Bulk modulus | 97.6 GPa[5] | ||||||||||||||||||||||||||||||
| Poisson ratio | 0.064–0.28[5] | ||||||||||||||||||||||||||||||
| Mohs hardness | 6.5 | ||||||||||||||||||||||||||||||
| CAS Number | 7440-21-3 | ||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||
| Naming | after Latin 'silex' or 'silicis', meaning flint | ||||||||||||||||||||||||||||||
| Prediction | Antoine Lavoisier (1787) | ||||||||||||||||||||||||||||||
| Discovery and first isolation | Jöns Jacob Berzelius[6][7] (1823) | ||||||||||||||||||||||||||||||
| Named by | Thomas Thomson (1817) | ||||||||||||||||||||||||||||||
| Main isotopes of silicon | |||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||
Silicon is a chemical element with symbol Si and atomic number 14. A hard and brittle crystalline solid with a blue-grey metallic lustre, it is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic table, along with carbon above it and germanium, tin, and lead below. It is rather unreactive, though less so than germanium, and has a very large chemical affinity for oxygen; as such, it was first prepared and characterized in pure form only in 1823 by Jöns Jakob Berzelius. Its melting and boiling points of 1414 °C and 3265 °C respectively are the second-highest among all the metalloids and nonmetals, being only surpassed by boron (carbon sublimes rather than melts at atmospheric pressure, albeit at a higher temperature than boron).
Silicon is the eighth most common element in the universe by mass, but very rarely occurs as the pure element in the Earth's crust. It is most widely distributed in dusts, sands, planetoids, and planets as various forms of silicon dioxide (silica) or silicates. Over 90% of the Earth's crust is composed of silicate minerals, making silicon the second most abundant element in the Earth's crust (about 28% by mass) after oxygen.
Most silicon is used commercially without being separated, and often with little processing of the natural minerals. Such use includes industrial construction with clays, silica sand, and stone. Silicates are used in Portland cement for mortar and stucco, and mixed with silica sand and gravel to make concrete for walkways, foundations, and roads. They are also used in whiteware ceramics such as porcelain, and in traditional quartz-based soda-lime glass and many other specialty glasses. Silicon compounds such as silicon carbide are used as abrasives and components of high-strength ceramics. Silicon is the basis of the widely used synthetic polymers called silicones.
Elemental silicon also has a large impact on the modern world economy. Most free silicon is used in the steel refining, aluminium-casting, and fine chemical industries (often to make fumed silica). Even more visibly, the relatively small portion of very highly purified elemental silicon used in semiconductor electronics (< 10%) is essential to integrated circuits — most computers, cell phones, and modern technology depend on it.
Silicon is an essential element in biology, although only traces are required by animals. However, various sea sponges and microorganisms, such as diatoms and radiolaria, secrete skeletal structures made of silica. Silica is deposited in many plant tissues, such as in the bark and wood of Chrysobalanaceae and the silica cells and silicified trichomes of Cannabis sativa, horsetails and many grasses.[8]
History
Jöns Jacob Berzelius, discoverer of silicon
In 1787 Antoine Lavoisier suspected that silica might be an oxide of a fundamental chemical element,[9] but the chemical affinity of silicon for oxygen is high enough that he had no means to reduce the oxide and isolate the element.[10] After an attempt to isolate silicon in 1808, Sir Humphry Davy proposed the name "silicium" for silicon, from the Latin silex, silicis for flint, and adding the "-ium" ending because he believed it to be a metal.[11] Most other languages use transliterated forms of Davy's name, sometimes adapted to local phonology (e.g. German Silizium, Turkish silisyum). A few others use instead a calque of the Latin root (e.g. Russian кремний, from кремень "flint"; Greek πυριτιο from πυρ "fire"; Finnish pii from piikivi "flint").[12]
In 1811, Gay-Lussac and Thénard are thought to have prepared impure amorphous silicon, through the heating of recently isolated potassium metal with silicon tetrafluoride, but they did not purify and characterize the product, nor identify it as a new element.[13] Silicon was given its present name in 1817 by Scottish chemist Thomas Thomson. He retained part of Davy's name but added "-on" because he believed that silicon was a nonmetal similar to boron and carbon.[14] In 1823, Jöns Jacob Berzelius prepared amorphous silicon using approximately the same method as Gay-Lussac (reducing potassium fluorosilicate with molten potassium metal), but purifying the product to a brown powder by repeatedly washing it.[15] As a result, he is usually given credit for the element's discovery.[16][17] The same year, Berzelius became the first to prepare silicon tetrachloride; silicon tetrafluoride had already been prepared long before in 1771 by Carl Wilhelm Scheele by dissolving silica in hydrofluoric acid.[10]
Silicon in its more common crystalline form was not prepared until 31 years later, by Deville.[18][19] By electrolyzing a mixture of sodium chloride and aluminium chloride containing approximately 10% silicon, he was able to obtain a slightly impure allotrope of silicon in 1854.[20] Later, more cost-effective methods have been developed to isolate several allotrope forms, the most recent being silicene in 2010.[21][22] Meanwhile, research on the chemistry of silicon continued; Friedrich Wöhler discovered the first volatile hydrides of silicon, synthesising trichlorosilane in 1857 and silane itself in 1858, but a detailed investigation of the silanes was only carried out in the early 20th century by Alfred Stock, despite early speculation on the matter dating as far back as the beginnings of synthetic organic chemistry in the 1830s.[23] Similarly, the first organosilicon compound, tetraethylsilane, was synthesised by Charles Friedel and James Crafts in 1863, but detailed characterisation of organosilicon chemistry was only done in the early 20th century by Frederick Kipping.[10]
Starting in the 1920s, the work of William Lawrence Bragg on X-ray crystallography successfully elucidated the compositions of the silicates, which had previously been known from analytical chemistry but had not yet been understood, together with Linus Pauling's development of crystal chemistry and Victor Goldschmidt's development of geochemistry. The middle of the 20th century saw the development of the chemistry and industrial use of siloxanes and the growing use of silicone polymers, elastomers, and resins. In the late 20th century, the complexity of the crystal chemistry of silicides was mapped, along with the solid-state chemistry of doped semiconductors.[10]
Because silicon is an important element in high-technology semiconductor devices, many places in the world bear its name. For example, Santa Clara Valley in California acquired the nickname Silicon Valley since the element is the base material used in the semiconductor industry located there. Since then, many other locations have been nicknamed for similar reasons.[24]
Characteristics
Physical and atomic
Silicon crystallizes in a diamond cubic crystal structure
A silicon atom has fourteen electrons. In the ground state, they are arranged in the electron configuration [Ne]3s23p2. Of these, four are valence electrons, occupying the 3s orbital and two of the 3p orbitals. Like the other members of its group, the lighter carbon and the heavier germanium, tin, and lead, it has the same number of valence electrons as valence orbitals: hence, it can complete its octet and obtain the stable noble gas configuration of argon by forming sp3 hybrid orbitals, forming tetrahedral SiX4 derivatives where the central silicon atom shares an electron pair with each of the four atoms it is bonded to.[25] The first four ionisation energies of silicon are 786.3, 1576.5, 3228.3, and 4354.4 kJ/mol respectively; these figures are high enough to preclude the possibility of simple cationic chemistry for the element. Following periodic trends, its single-bond covalent radius of 117.6 pm is intermediate between those of carbon (77.2 pm) and germanium (122.3 pm). The hexacoordinate ionic radius of silicon may be considered to be 40 pm, although this must be taken as a purely notional figure given the lack of a simple Si4+ cation in reality.[26]
At standard temperature and pressure, silicon is a shiny semiconductor with a bluish-grey metallic lustre; as typical for semiconductors, its resistivity drops as temperature rises. This arises because silicon has a small energy gap between its highest occupied energy levels (the valence band) and the lowest unoccupied ones (the conduction band). The Fermi level is about halfway between the valence and conduction bands and is the energy at which a state is as likely to be occupied by an electron as not. Hence pure silicon is an insulator at room temperature. However, doping silicon with a pnictogen such as phosphorus, arsenic, or antimony introduces one extra electron per dopant and these may then be excited into the conduction band either thermally or photolytically, creating an n-type semiconductor. Similarly, doping silicon with a group 13 element such as boron, aluminium, and gallium results in the introduction of acceptor levels that trap electrons that may be excited from the filled valence band, creating a p-type semiconductor. Joining n-type silicon to p-type silicon creates a p-n junction with a common Fermi level; electrons flow from n to p, while holes flow from p to n, creating a voltage drop. This p-n junction thus acts as a diode that can rectify alternating current that allows current to pass more easily one way than the other. A transistor is an n-p-n junction, with a thin layer of weakly p-type silicon between two n-type regions. Biasing the emitter through a small forward voltage and the collector through a large reverse voltage allows the transistor to act as a triode amplifier.[27]
Silicon crystallises in a giant covalent structure at standard conditions, specifically in a diamond cubic lattice. It thus has a high melting point of 1414 °C, as a lot of energy is required to break the strong covalent bonds and melt the solid. It is not known to have any allotropes at standard pressure, but several other crystal structures are known at higher pressures. The general trend is one of increasing coordination number with pressure, culminating in a hexagonal close-packed allotrope at about 40 gigapascals known as Si–VII (the standard modification being Si–I). Silicon boils at 3265 °C: this, while high, is still lower than the temperature at which its lighter congener carbon sublimes (3642 °C) and silicon similarly has a lower heat of vaporisation than carbon, consistent with the fact that the Si–Si bond is weaker than the C–C bond.[27]
Isotopes
Naturally occurring silicon is composed of three stable isotopes, 28Si (92.23%), 29Si (4.67%), and 30Si (3.10%).[28] Out of these, only 29Si is of use in NMR and EPR spectroscopy,[29] as it is the only one with a nuclear spin (I = 1/2).[30] All three are produced in stars through the oxygen-burning process, with 28Si being made as part of the alpha process and hence the most abundant. The fusion of 28Si with alpha particles by photodisintegration rearrangement in stars is known as the silicon-burning process; it is the last stage of stellar nucleosynthesis before the rapid collapse and violent explosion of the star in question in a type II supernova.[31]Twenty radioisotopes have been characterized, with the two stablest being 32Si with a half-life of about 150 years, and 31Si with a half-life of 2.62 hours.[28] All of the remaining radioactive isotopes have half-lives that are less than seven seconds, and the majority of these have half-lives that are less than one tenth of a second.[28] Silicon does not have any known nuclear isomers.[28] 32Si undergoes low-energy beta decay to 32P and then stable 32S. 31Si may be produced by the neutron activation of natural silicon and is thus useful for quantitative analysis; it can be easily detected by its characteristic beta decay to stable 31P, in which the emitted electron carries up to 1.48 MeV of energy.[30]
The known isotopes of silicon range in mass number from 22 to 44.[28] The most common decay mode of the isotopes with mass numbers lower than the three stable isotopes is inverse beta decay, primarily forming aluminium isotopes (13 protons) as decay products.[28] The most common decay mode for the heavier unstable isotopes is beta decay, primarily forming phosphorus isotopes (15 protons) as decay products.[28]
Chemistry and compounds
Crystalline bulk silicon is rather inert, but becomes more reactive at high temperatures. Like its neighbour aluminium, silicon forms a thin, continuous surface layer of silicon dioxide (SiO2) that protects the metal from oxidation. Thus silicon does not measurably react with the air below 900 °C, but formation of the vitreous dioxide rapidly increases between 950 °C and 1160 °C and when 1400 °C is reached, atmospheric nitrogen also reacts to give the nitrides SiN and Si3N4. Silicon reacts with gaseous sulfur at 600 °C and gaseous phosphorus at 1000 °C. This oxide layer nevertheless does not prevent reaction with the halogens; fluorine attacks silicon vigorously at room temperature, chlorine does so at about 300 °C, and bromine and iodine at about 500 °C. Silicon does not react with most aqueous acids, but is oxidised and fluorinated by a mixture of concentrated nitric acid and hydrofluoric acid; it readily dissolves in hot aqueous alkali to form silicates. At high temperatures, silicon also reacts with alkyl halides; this reaction can be catalysed by copper to directly synthesise organosilicon chlorides as precursors to silicone polymers. Upon melting, silicon becomes extremely reactive, alloying with most metals to form silicides, and reducing most metal oxides because the heat of formation of silicon dioxide is so large. As a result, containers for liquid silicon must be made of refractory, unreactive materials such as zirconium dioxide or group 4, 5, and 6 borides.[27]Tetrahedral coordination is a major structural motif in silicon chemistry just as it is for carbon chemistry. However, the 3p subshell is rather more diffuse than the 2p subshell and does not hybridise as well with the 3s subshell. As a result, the chemistry of silicon and its heavier congeners shows significant differences from that of carbon,[32] and thus octahedral coordination is also significant.[27] For example, the electronegativity of silicon (1.90) is much less than that of carbon (2.55), because the valence electrons of silicon are further from the nucleus than those of carbon and hence experience smaller electrostatic forces of attraction from the nucleus. The poor overlap of 3p orbitals also results in a much lower tendency towards catenation (formation of Si–Si bonds) for silicon than for carbon due to the concomitant weakening of the Si–Si bond compared to the C–C bond:[33] the average Si–Si bond energy is approximately 226 kJ/mol, compared to a value of 356 kJ/mol for the C–C bond.[34] This results in multiply bonded silicon compounds generally being much less stable than their carbon counterparts, an example of the double bond rule. On the other hand, the presence of 3d orbitals in the valence shell of silicon suggests the possibility of hypervalence, as seen in five- and six-coordinate derivatives of silicon such as SiX−
5 and SiF2−
6.[33] Lastly, because of the increasing energy gap between the valence s and p orbitals as the group is descended, the divalent state grows in importance from carbon to lead, so that a few unstable divalent compounds are known for silicon; this lowering of the main oxidation state, in tandem with increasing atomic radii, results in an increase of metallic character down the group. Silicon already shows some incipient metallic behaviour, particularly in the behaviour of its oxide compounds and its reaction with acids as well as bases (though this takes some effort), and is hence often referred to as a metalloid rather than a nonmetal.[33] However, metallicity does not become clear in group 14 until germanium and dominant until tin, with the growing importance of the lower +2 oxidation state.[10]
Silicon shows clear differences with carbon. For example, organic chemistry has very few analogies with silicon chemistry, while silicate minerals have a structural complexity unseen in oxocarbons.[10] Silicon tends to resemble germanium far more than it does carbon, and this resemblance is enhanced by the d-block contraction resulting in the size of the germanium atom being much closer to that of the silicon atom than periodic trends would predict.[26] Nevertheless, there are still some differences because of the growing importance of the divalent state in germanium compared to silicon, that result in germanium being significantly more metallic than silicon. Additionally, the lower Ge–O bond strength compared to the Si–O bond strength results in the absence of "germanone" polymers that would be analogous to silicone polymers.[34]
Silicides
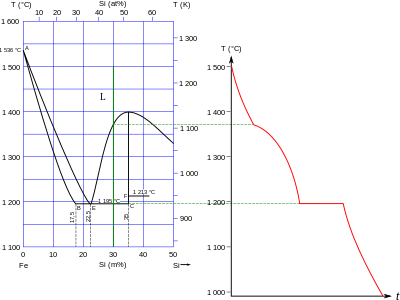
Phase diagram of the Fe–Si system
Many metal silicides are known, most of which have formulae that cannot be explained through simple appeals to valence: their bonding ranges from metallic to ionic and covalent. Some known stoichiometries are M6Si, M5Si, M4Si, M15Si4, M3Si, M5Si2, M2Si, M5Si3, M3Si2, MSi, M2Si3, MSi2, MSi3, and MSi6. They are structurally more similar to the borides than the carbides, in keeping with the diagonal relationship between boron and silicon, although the larger size of silicon than boron means that exact structural analogies are few and far between. The heats of formation of the silicides are usually similar to those of the borides and carbides of the same elements, but they usually melt at lower temperatures.[35] Silicides are known for all stable elements in groups 1–10, with the exception of beryllium: in particular, uranium and the transition metals of groups 4–10 show the widest range of stoichiometries. Except for copper, the metals in groups 11–15 do not form silicides. Most instead form eutectic mixtures, although the heaviest post-transition metals mercury, thallium, lead, and bismuth are completely immiscible with liquid silicon.[35]
Silicides are usually prepared by direct reaction of the elements. For example, the alkali metals and alkaline earth metals react with silicon or silicon oxide to give silicides. Nevertheless, even with these highly electropositive elements true silicon anions are not obtainable, and most of these compounds are semiconductors. For example, the alkali metal silicides (M+)
4(E4−
4) contain pyramidal tricoordinate silicon in the Si4−
4 anion, isoeelctronic with white phosphorus, P4.[35][36] Metal-rich silicides tend to have isolated silicon atoms (e.g. Cu5Si); with increasing silicon content, catenation increases, resulting in isolated clusters of two (e.g. U3Si2) or four silicon atoms (e.g. [K+]4[Si4]4−) at first, followed by chains (e.g. CaSi), layers (e.g. CaSi2), or three-dimensional networks of silicon atoms spanning space (e.g. α-ThSi2) as the silicon content rises even higher.[35]
The silicides of the group 1 and 2 metals are usually more reactive than the transition metal silicides. The latter usually do not react with aqueous reagents, except for hydrofluoric acid; however, they do react with much more aggressive reagents like liquid potassium hydroxide, or gaseous fluorine or chlorine when red-hot. The pre-transition metal silicides instead readily react with water and aqueous acids, usually producing hydrogen or silanes:[35]
- Na2Si + 3 H2O → Na2SiO3 + 3 H2
- Mg2Si + 2 H2SO4 → 2 MgSO4 + SiH4
Silanes
Speculation on silicon hydride chemistry started from the 1830s, contemporary with the development of synthetic organic chemistry. Silane itself, as well as trichlorosilane, were first synthesised by Friedrich Wöhler and Heinrich Buff in 1857 by reacting aluminium–silicon alloys with hydrochloric acid, and characterised as SiH4 and SiHCl3 by Charles Friedel and Albert Ladenburg in 1867. Disilane (Si2H6) followed in 1902, when it was first made by Henri Moissan and Samuel Smiles by the protonolysis of magnesium silicides. Further investigation had to wait until 1916 because of the great reactivity and thermal instability of the silanes; it was then that Alfred Stock began the study of silicon hydrides in earnest with new greaseless vacuum techniques, as they were found as contaminants of his focus, the boron hydrides. The names silanes and boranes are due to him, based on analogy with the alkanes.[23][37][38] Moissan and Smiles' method of preparation of silanes and silane derivatives via protonolysis of metal silicides is still used, although the yield is lowered by the hydrolysis of the products that occurs simultaneously, and thus the preferred route today is to treat substituted silanes with hydride reducing agents such as lithium aluminium hydride in etheric solutions at low temperatures. Direct reaction of HX or RX with silicon, possibly with a catalyst such as copper, is also a viable method to produce substituted silanes.[23]The silanes comprise a homologous series of silicon hydrides with the general formula SinH2n + 2. They are all strong reducing agents. Unbranched and branched trains are known up to n = 8, and the cycles Si5H10 and Si6H12 are also known. The first two, silane and disilane, are colourless gases; the heavier members of the series are volatile liquids. All silanes are very reactive and catch fire or explode spontaneously in air. They become less thermally stable with room temperature, so that only silane is indefinitely stable at room temperature, although disilane does not decompose very quickly (only 2.5% of a sample decomposes after eight months have passed).[23] They decompose to form polymeric polysilicon hydride and hydrogen gas.[39][40] As expected from the difference in atomic weight, the silanes are less volatile than the corresponding alkanes and boranes, but more volatile than the corresponding germanes. They are much more reactive than the corresponding alkanes, because the larger radius of silicon compared to carbon facilitates nucleophilic attack at the silicon, the greater polarity of the Si–H bond compared to the C–H bond, and the ability of silicon to expand its octet and hence form adducts and lower the reaction's activation energy.[23]
Silane pyrolysis gives polymeric species and finally elemental silicon and hydrogen; indeed ultrapure silicon is commercially produced by the pyrolysis of silane. While the thermal decomposition of alkanes starts by the breaking of a C–H or C–C bond and the formation of radical intermediates, polysilanes decompose by eliminating silenes :SiH2 or :SiHR, as the activation energy of this process (~210 kJ/mol) is much less than the Si–Si and Si–H bond energies. While pure silanes do not react with pure water or dilute acids, traces of alkali catalyse immediate hydrolysis to hydrated silicon dioxide. If the reaction is carried out in methanol, controlled solvolysis results in the products SiH2(OMe)2, SiH(OMe)3, and Si(OMe)4. The Si–H bond also adds to alkenes, a reaction which proceeds slowly and speeds up with increasing substitution of the silane involved. At 450 °C, silane participates in an addition reaction with acetone, as well as a ring-opening reaction with ethylene oxide. Direct reaction of the silanes with chlorine or bromine results in explosions at room temperature, but the reaction of silane with bromine at −80 °C is controlled and yields bromosilane and dibromosilane. The monohalosilanes may be formed by reacting silane with the appropriate hydrogen halide with an Al2X6 catalyst, or by reacting silane with a solid silver halide in a heated flow reactor:[23]
- SiH4 + 2 AgCl SiH3Cl + HCl + 2 Ag
3 anions in the NaCl structure, and is made by the reduction of silane by potassium metal.[41] Additionally, the reactive hypervalent species SiH−
5 is also known.[23] With suitable organic substituents it is possible to produce stable polysilanes: they have surprisingly high electric conductivities, arising from sigma delocalisation of the electrons in the chain.[42]
Halides
Silicon and silicon carbide readily react with all four stable halogens, forming the colourless, reactive and volatile silicon tetrahalides.[43] Silicon tetrafluoride may also be made by fluorinating the other silicon halides, and is produced by the attack of hydrofluoric acid on glass.[44] Heating two different tetrahalides together also produce a random mixture of mixed halides, which may also be produced by halogen exchange reactions. The melting and boiling points of these species usually rise with increasing atomic weight, though there are many exceptions: for example, the melting and boiling points drop as one passes from SiFBr3 through SiFClBr2 to SiFCl2Br. The shift from the hypoelectronic elements in group 13 and earlier to the group 14 elements is illustrated by the change from an infinite ionic structure in aluminium fluoride to a lattice of simple covalent silicon tetrafluoride molecules, as dictated by the lower electronegativity of aluminium than silicon, the stoichiometry (the +4 oxidation state being too high for true ionicity), and the smaller size of the silicon atom compared to the aluminium atom.[43] Silicon tetrachloride is manufactured on a huge scale as a precursor to the production of pure silicon, silicon dioxide, and some silicon esters.[43] The silicon tetrahalides hydrolyse readily in water, unlike the carbon tetrahalides, again because of the larger size of the silicon atom rendering it more open to nucleophilic attack and the ability of the silicon atom to expand its octet which carbon lacks.[44] The reaction of silicon fluoride with excess hydrofluoric acid produces the octahedral hexafluorosilicate anion SiF2−6.[44]
Analogous to the silanes, halopolysilanes SinX2n + 2 are also known. While catenation in carbon compounds is maximised in the hydrogen compounds rather than the halides, the opposite is true for silicon, so that the halopolysilanes are known up to at least Si14F30, Si6Cl14, and Si4Br10. A suggested explanation for this phenomenon is the compensation for the electron loss of silicon to the more electronegative halogen atoms by pi backbonding from the filled pπ orbitals on the halogen atoms to the empty dπ orbitals on silicon: this is similar to the situation of carbon monoxide in metal carbonyl complexes and explains their stability. These halopolysilanes may be produced by comproportionation of silicon tetrahalides with elemental silicon, or by condensation of lighter halopolysilanes (trimethylammonium being a useful catalyst for this reaction).[43]
Silica
Silicon dioxide (SiO2), also known as silica, is one of the most well-studied compounds, second only to water. Twelve different crystal modifications of silica are known, the most common being α-quartz, a major constituent of many rocks such as granite and sandstone. It is also known to occur pure as rock crystal; impure forms are known as rose quartz, smoky quartz, morion, amethyst, and citrine. Some poorly crystalline forms of quartz are also known, such as chalcedony, chrysoprase, carnelian, agate, onyx, jasper, heliotrope, and flint. Other modifications of silicon dioxide are known in some other minerals such as tridymite and cristobalite, as well as the much less common coesite and stishovite. Biologically generated forms are also known as kieselguhr and diatomaceous earth. Vitreous silicon dioxide is known as tektites, and obsidian, and rarely as lechatelierite. Some synthetic forms are known as keatite and W-silica. Opals are composed of complicated crystalline aggregates of partially hydrated silicon dioxide.[45]
Condensed polysilicic acid
Silica is rather inert chemically. It is not attacked by any acids other than hydrofluoric acid. However, it slowly dissolves in hot concentrated alkalis, and does so rather quickly in fused metal hydroxides or carbonates to give metal silicates. Among the elements, it is attacked only by fluorine at room temperature to form silicon tetrafluoride: hydrogen and carbon also react, but require temperatures over 1000 °C to do so. Silica nevertheless reacts with many metal and metalloid oxides to form a wide variety of compounds important in the glass and ceramic industries above all, but also have many other uses: for example, sodium silicate is often used in detergents due to its buffering, saponifying, and emulsifying properties.[45]
Silicic acids
Adding water to silica drops its melting point by around 800 °C due to the breaking of the structure by replacing Si–O–Si linkages with terminating Si–OH groups. Increasing water concentration results in the formation of hydrated silica gels and colloidal silica dispersions. Many hydrates and silicic acids exist in the most dilute of aqueous solutions, but these are rather insoluble and quickly precipitate and condense and cross-link to form various polysilicic acids of variable combinations following the formula [SiOx(OH)4−2x]n, similar to the behaviour of boron, aluminium, and iron, among other elements. Hence, although some simple silicic acids have been identified in dilute solutions, such as orthosilicic acid Si(OH)4 and metasilicic acid SiO(OH)2, none of these are likely to exist in the solid state.[45]Silicate minerals
About 95% of the Earth's crustal rocks are made of silica or silicate and aluminosilicate minerals, as reflected in oxygen, silicon, and aluminium being the three most common elements in the crust (in that order).[46] Measured by mass, silicon makes up 27.7% of the Earth's crust.[47] Pure silicon crystals are very rarely found in nature, but notable exceptions are crystals as large as to 0.3 mm across found during sampling gases from the Kudriavy volcano on the island of Iturup, one of the Kuril Islands.[48][49]Silicate and aluminosilicate minerals have many different structures and varying stoichiometry, but they may be classified following some general principles. Tetrahedral {SiO4} units are common to almost all these compounds, either as discrete structures, or combined into larger units by the sharing of corner oxygen atoms. These may be divided into neso-silicates (discrete {SiO4} units) sharing no oxygen atoms, soro-silicates (discrete {Si2O7} units) sharing one, cyclo-silicates (closed ring structures) and ino-silicates (continuous chain or ribbon structures) both sharing two, phyllo-silicates (continuous sheets) sharing three, and tecto-silicates (continuous three-dimensional frameworks) sharing four. The lattice of oxygen atoms that results is usually close-packed or close to it, with the charge being balanced by other cations in various different polyhedral sites according to size.[46]
The orthosilicates MII
2SiO
4 (M = Be, Mg, Mn, Fe, Zn) and ZrSiO4 are neso-silicates. Be2SiO4 (phenacite) is rather unusual as both BeII and SiIV occupy tetrahedral four-coordinated sites; the other divalent cations instead occupy six-coordinated octahedral sites and often isomorphously replace each other as in olivine, (Mg,Fe,Mn)2SiO4. Zircon, ZrSiO4, demands eight-coordination of the ZrIV cations due to stoichiometry and because of their larger ionic radius (84 pm). Also significant are the garnets, [MII
3MIII
2(SiO
4)
3], in which the divalent cations (e.g. Ca, Mg, Fe) are eight-coordinated and the trivalent ones are six-coordinated (e.g. Al, Cr, Fe). Regular coordination is not always present: for example, it is not found in Ca2SiO4, which mixes six- and eight-coordinate sites for CaII. Soro-silicates, involving discrete double or triple tetrahedral units, are quite rare: metasilicates involving cyclic "[(SiO3)n]2n−" units of corner-abutting tetrahedra forming a polygonal ring are also known.[46]
Chain metasilicates, {SiO2−
3}
∞, form by the corner-sharing of an indefinite chain of linked {SiO4} tetrahedra. Many differences arise due to the differing repeat distances of conformation across the line of tetrahedra. A repeat distance of two is most common, as in most pyroxene minerals, but repeat distances of one, three, four, five, six, seven, nine, and twelve are also known. These chains can then link across each other to form double chains and ribbons, as in the asbestos minerals, involving repeated chains of cyclic tetrahedron rings.[46]
A typical zeolite structure
Layer silicates, such as the clay minerals and the micas, are very common, and are often formed by horizontal cross-linking of metasilicate chains or planar condensation of smaller units. An example is kaolinite [Al2(OH)4Si2O5]; in many of these minerals cation and anion replacement is common, so that for example tetrahedral SiIV may be replaced by AlIII, octahedral AlIII by MgII, and OH− by F−. Three-dimensional framework aluminosilicates are structurally very complex; they may be conceived of as starting from the SiO2 structure, but having replaced up to one-half of the SiIV atoms with AlIII they require more cations to be included in the structure to balance charge. Examples include feldspars (the most abundant minerals on the Earth), zeolites, and ultramarines. Many feldspars can be thought of as forming part of the ternary system NaAlSi3O8–KAlSi3O8–CaAl2Si2O8. Their lattice is destroyed by high pressure prompting AlIII to undergo six-coordination rather than four-coordination, and this reaction destroying feldspars may be a reason for the Mohorovičić discontinuity, which would imply that the crust and mantle have the same chemical composition but different lattices, although this is not a universally held view. Zeolites have many polyhedral cavities in their frameworks (truncated cuboctahedra being most common, but other polyhedra are also known as zeolite cavities), allowing them to include loosely bound molecules such as water in their structure. Ultramarines alternate silicon and aluminium atoms and include a variety of other anions such as Cl−, SO2−
4, and S2−
2, but are otherwise similar to the feldspars.[46]
Other inorganic compounds
Silicon disulfide (SiS2) is formed by burning silicon in gaseous sulfur at 100 °C; sublimation of the resulting compound in nitrogen results in white, flexible long fibres reminiscent of asbestos with a structure similar to W-silica. This melts at 1090 °C and sublimes at 1250 °C; at high temperature and pressure this transforms to a crystal structure analogous to cristobalite. However, SiS2 lacks the variety of structures of SiO2, and quickly hydrolyses to silica and hydrogen sulfide. It is also ammonoloysed quickly and completely by liquid ammonia as follows to form an imide:[50]- SiS2 + 4 NH3 → Si(NH)2 + 2 NH4SH
Silicon nitride, Si3N4, can be formed by directly reacting silicon with nitrogen above 1300 °C, but a more economical means of production is by heating silica and coke in a stream of nitrogen and hydrogen gas at 1500 °C. It would make a promising ceramic if not for the difficulty of working with and sintering it: it is chemically near-totally inert, and even above 1000 °C it keeps its strength, shape, and continues to be resistant to wear and corrosion. It is very hard (9 on the Mohs hardness scale), dissociates only at 1900 °C at 1 atm, and is quite dense (density 3.185 g/cm3), because of its compact structure similar to that of phenacite (Be2SiO4). A similar refractory material is Si2N2O, formed by heating silicon and silica at 1450 °C in an argon stream containing 5% nitrogen gas, involving 4-coordinate silicon and 3-coordinate nitrogen alternating in puckered hexagonal tilings interlinked by non-linear Si–O–Si linkages to each other.[50]
Reacting silyl halides with ammonia or alkylammonia derivatives in the gaseous phase or in ethanolic solution produces various volatile silylamides, which are silicon analogues of the amines:[50]
- 3 SiH3Cl + 4 NH3 → N(SiH3)3 + 3 NH4Cl
- SiH3Br + 2 Me2NH → SiH3NMe2 + Me2NH2Br
- 4 SiH3I + 5 N2H4 → (SiH3)2NN(SiH3)2 + 4 N2H5I
Silicon carbide
Silicon carbide (SiC) was first made by Edward Goodrich Acheson in 1891, who named it carborundum to reference its intermediate hardness and abrasive power between diamond (an allotrope of carbon) and corundum (aluminium oxide). He soon founded a company to manufacture it, and today about one million tonnes are produced each year.[51] Silicon carbide exists in about 250 crystalline forms.[52] The polymorphism of SiC is characterized by a large family of similar crystalline structures called polytypes. They are variations of the same chemical compound that are identical in two dimensions and differ in the third. Thus, they can be viewed as layers stacked in a certain sequence.[53] It is made industrially by reduction of quartz sand with excess coke or anthracite at 2000–2500 °C in an electric furnace:[51]
- SiO2 + 2 C → Si + 2 CO
- Si + C → SiC
Organosilicon compounds

A hydrosilylation reaction, in which Si–H is added to an unsaturated substrate
Because the Si–C bond is close in strength to the C–C bond, organosilicon compounds tend to be markedly thermally and chemically stable. For example, tetraphenylsilane (SiPh4) may be distilled in air even at its boiling point of 428 °C, and so can its substituted derivatives Ph3SiCl and Ph2SiCl2, which boil at 378 °C and 305 °C respectively. Furthermore, since carbon and silicon are chemical congeners, organosilicon chemistry shows some significant similarities with carbon chemistry, for example in the propensity of such compounds for catenation and forming multiple bonds.[54] However, significant differences also arise: since silicon is more electropositive than carbon, bonds to more electronegative elements are generally stronger with silicon than with carbon, and vice versa. Thus the Si–F bond is significantly stronger than even the C–F bond and is one of the strongest single bonds, while the Si–H bond is much weaker than the C–H bond and is readily broken. Furthermore, the ability of silicon to expand its octet is not shared by carbon, and hence some organosilicon reactions have no organic analogues. For example, nucleophilic attack on silicon does not proceed by the SN2 or SN1 processes, but instead goes through a negatively charged true pentacoordinate intermediate and appears like a substitution at a hindered tertiary atom. This works for silicon, unlike for carbon, because the long Si–C bonds reduce the steric hindrance and the d-orbital of silicon is geometrically unconstrained for nucleophilic attack, unlike for example a C–O σ* antibonding orbital. Nevertheless, despite these differences, the mechanism is still often called "SN2 at silicon" for simplicity.[55]
One of the most useful silicon-containing groups is trimethylsilyl, Me3Si–. The Si–C bond connecting it to the rest of the molecule is reasonably strong, allowing it to remain while the rest of the molecule undergoes reactions, but is not so strong that it cannot be removed specifically when needed, for example by the fluoride ion, which is a very weak nucleophile for carbon compounds but a very strong one for organosilicon compounds. It may be compared to acidic protons; while trisilylmethyl is removed by hard nucleophiles instead of bases, both removals usually promote elimination. As a general rule, while saturated carbon is best attacked by nucleophiles that are neutral compounds, those based on nonmetals far down on the periodic table (e.g. sulfur, selenium, or iodine), or even both, silicon is best attacked by charged nucleophiles, particularly those involving such highly electronegative nonmetals as oxygen, fluorine, or chlorine. For example, enolates react at the carbon in haloalkanes, but at the oxygen in silyl chlorides; and when trimethylsilyl is removed from an organic molecule using hydroxide as a nucleophile, the product of the reaction is not the silanol as one would expect from using carbon chemistry as an analogy, because the siloxide is strongly nucleophilic and attacks the original molecule to yield the silyl ether hexamethyldisiloxane, (Me3Si)2O. Conversely, while the SN2 reaction is mostly unaffected by the presence of a partial positive charge (δ+) at the carbon, the analogous "SN2" reaction at silicon is so affected. Thus, for example, the silyl triflates are so electrophilic that they react 108 to 109 times faster than silyl chlorides with oxygen-containing nucleophiles. Trimethylsilyl triflate is in particular a very good Lewis acid and is used to convert carbonyl compounds to acetals and silyl enol ethers, reacting them together analogously to the aldol reaction.[55]
Si–C bonds are commonly formed in three ways. In the laboratory, preparation is often carried out in small quantities by reacting tetrachlorosilane with organolithium, Grignard, or organoaluminium reagents, or by catalytic addition of Si–H across C=C double bonds. The second route has the drawback of not being applicable to the most important silanes, the methyl and phenyl silanes. Organosilanes are made industrially by directly reacting alkyl or aryl halides with silicon with 10% by weight metallic copper as a catalyst. Standard organic reactions suffice to produce many derivatives; the resulting organosilanes are often significantly more reactive than their carbon congeners, readily undergoing hydrolysis, ammonolysis, alcoholysis, and condensation to form cyclic oligomers or linear polymers.[54]
Silicone polymers
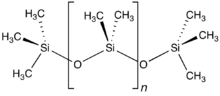
Structure of polydimethylsiloxane, the principal component of silicones
The word "silicone" was first used by Frederick Kipping in 1901. He invented the word to illustrate the similarity of chemical formulae between Ph2SiO and benzophenone, Ph2CO, although he also stressed the lack of chemical resemblance due to the polymeric structure of Ph2SiO, which is not shared by Ph2CO.[54]
Silicones may be considered analogous to mineral silicates, in which the methyl groups of the silicones correspond to the isoelectronic O− of the silicates.[54] They are quite stable to extreme temperatures, oxidation, and water, and have useful dielectric, antistick, and antifoam properties. Furthermore, they are resistant over long periods of time to ultraviolet radiation and weathering, and are inert physiologically. They are fairly unreactive, but do react with concentrated solutions bearing the hydroxide ion and fluorinating agents, and occasionally can be even used as mild reagents for selective syntheses. For example, (Me3Si)2O is valuable for the preparation of derivatives of molybdenum and tungsten oxyhalides, converting a tungsten hexachloride suspension in dichloroethane solution quantitatively to WOCl4 in under an hour at room temperature, and then to yellow WO2Cl2 at 100 °C in light petroleum at a yield of 95% overnight.[54]
Occurrence
Olivine
In the universe, silicon is the seventh most abundant element, coming after hydrogen, helium, carbon, nitrogen, oxygen, and neon. These abundances are not replicated well on Earth due to substantial separation of the elements taking place during the formation of the Solar System. Silicon makes up 27.2% of the Earth's crust by weight, second only to oxygen at 45.5%, with which it is always associated in nature. Further fractionation took place in the formation of the Earth by planetary differentiation: Earth's core, which makes up 31.5% of the mass of the Earth, has approximate composition Fe25Ni2Co0.1S3; the mantle makes up 68.1% of the Earth's mass and is composed mostly of denser oxides and silicates, an example being olivine, (Mg,Fe)2SiO4; while the lighter siliceous minerals such as aluminosilicates rise to the surface and form the crust, making up 0.4% of the Earth's mass.[56]
The crystallisation of igneous rocks from magma depends on a number of factors; among them are the chemical composition of the magma, the cooling rate, and some properties of the individual minerals to be formed, such as lattice energy, melting point, and complexity of their crystal structure. As magma is cooled, olivine appears first, followed by pyroxene, amphibole, biotite mica, orthoclase feldspar, muscovite mica, quartz, zeolites, and finally hydrothermal minerals. This sequence shows a trend towards increasingly complex silicate units with cooling, and the introduction of hydroxide and fluoride anions in addition to oxides. Many metals can substitute for silicon. After these igneous rocks undergo weathering, transport, and deposition, sedimentary rocks like clay, shale, and sandstone are formed. Metamorphism also can occur at high temperatures and pressures, creating an even vaster variety of minerals.[56]
Production
Silicon of 96–99% purity is made by reducing quartzite or sand with highly pure coke. The reduction is carried out in an electric arc furnace, with an excess of SiO2 used to stop silicon carbide (SiC) from accumulating:[30]- SiO2 + 2 C → Si + 2 CO
- 2 SiC + SiO2 → 3 Si + 2 CO
Ferrosilicon alloy
This reaction, known as carbothermal reduction of silicon dioxide, is usually conducted in the presence of scrap iron with low amounts of phosphorus and sulfur, produing ferrosilicon.[30] Ferrosilicon, an iron-silicon alloy that contains varying ratios of elemental silicon and iron, accounts for about 80% of the world's production of elemental silicon, with China, the leading supplier of elemental silicon, providing 4.6 million tonnes (or 2/3 of the world output) of silicon, most of which is in the form of ferrosilicon. It is followed by Russia (610,000 t), Norway (330,000 t), Brazil (240,000 t) and the United States (170,000 t).[57] Ferrosilicon is primarily used by the iron and steel industry (see below) with primary use as alloying addition in iron or steel and for de-oxidation of steel in integrated steel plants.[30] Another sometimes used reaction is aluminothermal reduction of silicon dioxide, as follows:[58]
- 3 SiO2 + 4 Al → 3 Si + 2 Al2O3
Applications
Compounds
Most silicon is used industrially without being purified, and indeed often with comparatively little processing from its natural form. Over 90% of the Earth's crust is composed of silicate minerals, which are compounds of silicon and oxygen, often with metallic ions when negatively charged silicate anions require cations to balance the charge. Many of these have direct commercial uses, such as clays, silica sand and most kinds of building stone. Thus, the vast majority of uses for silicon are as structural compounds, either as the silicate minerals or silica (crude silicon dioxide). Silicates are used in making Portland cement (made mostly of calcium silicates) which is used in building mortar and modern stucco, but more importantly, combined with silica sand, and gravel (usually containing silicate minerals like granite), to make the concrete that is the basis of most of the very largest industrial building projects of the modern world.[59]Silica is used to make fire brick, a type of ceramic. Silicate minerals are also in whiteware ceramics, an important class of products usually containing various types of fired clay minerals (natural aluminium phyllosilicates). An example is porcelain which is based on the silicate mineral kaolinite. Traditional glass (silica-based soda-lime glass) also functions in many of the same ways, and is also used for windows and containers. In addition, specialty silica based glass fibers are used for optical fiber, as well as to produce fiberglass for structural support and glass wool for thermal insulation.
Silicones are often used in waterproofing treatments, molding compounds, mold-release agents, mechanical seals, high temperature greases and waxes, and caulking compounds. Silicone is also sometimes used in breast implants, contact lenses, explosives and pyrotechnics.[60] Silly Putty was originally made by adding boric acid to silicone oil.[61] Other silicon compounds function as high-technology abrasives and new high-strength ceramics based upon silicon carbide. Silicon is a component of some superalloys.
Alloys
Elemental silicon is added to molten cast iron as ferrosilicon or silicocalcium alloys to improve performance in casting thin sections and to prevent the formation of cementite where exposed to outside air. The presence of elemental silicon in molten iron acts as a sink for oxygen, so that the steel carbon content, which must be kept within narrow limits for each type of steel, can be more closely controlled. Ferrosilicon production and use is a monitor of the steel industry, and although this form of elemental silicon is grossly impure, it accounts for 80% of the world's use of free silicon. Silicon is an important constituent of electrical steel, modifying its resistivity and ferromagnetic properties.The properties of silicon can be used to modify alloys with metals other than iron. "Metallurgical grade" silicon is silicon of 95–99% purity. About 55% of the world consumption of metallurgical purity silicon goes for production of aluminium-silicon alloys (silumin alloys) for aluminium part casts, mainly for use in the automotive industry. Silicon's importance in aluminium casting is that a significantly high amount (12%) of silicon in aluminium forms a eutectic mixture which solidifies with very little thermal contraction. This greatly reduces tearing and cracks formed from stress as casting alloys cool to solidity. Silicon also significantly improves the hardness and thus wear-resistance of aluminium.[62][63]
Electronics
Silicon wafer with mirror finish
Most elemental silicon produced remains as a ferrosilicon alloy, and only about 20% is refined to metallurgical grade purity (a total of 1.3–1.5 million metric tons/year). An estimated 15% of the world production of metallurgical grade silicon is further refined to semiconductor purity.[63] This typically is the "nine-9" or 99.9999999% purity [64] nearly defect-free single crystalline material.[65]
Monocrystalline silicon of such purity is usually produced by the Czochralski process, is used to produce silicon wafers used in the semiconductor industry, in electronics, and in some high-cost and high-efficiency photovoltaic applications.[66] Pure silicon is an intrinsic semiconductor, which means that unlike metals, it conducts electron holes and electrons released from atoms by heat; silicon's electrical conductivity increases with higher temperatures. Pure silicon has too low a conductivity (i.e., too high a resistivity) to be used as a circuit element in electronics. In practice, pure silicon is doped with small concentrations of certain other elements, which greatly increase its conductivity and adjust its electrical response by controlling the number and charge (positive or negative) of activated carriers. Such control is necessary for transistors, solar cells, semiconductor detectors, and other semiconductor devices used in the computer industry and other technical applications.[67] In silicon photonics, silicon can be used as a continuous wave Raman laser medium to produce coherent light.[68]
In common integrated circuits, a wafer of monocrystalline silicon serves as a mechanical support for the circuits, which are created by doping and insulated from each other by thin layers of silicon oxide, an insulator that is easily produced by exposing the element to oxygen under the proper conditions. Silicon has become the most popular material for both high power semiconductors and integrated circuits because it can withstand the highest temperatures and greatest electrical activity without suffering avalanche breakdown (an electron avalanche is created when heat produces free electrons and holes, which in turn pass more current, which produces more heat). In addition, the insulating oxide of silicon is not soluble in water, which gives it an advantage over germanium (an element with similar properties which can also be used in semiconductor devices) in certain fabrication techniques.[69]
Monocrystalline silicon is expensive to produce, and is usually justified only in production of integrated circuits, where tiny crystal imperfections can interfere with tiny circuit paths. For other uses, other types of pure silicon may be employed. These include hydrogenated amorphous silicon and upgraded metallurgical-grade silicon (UMG-Si) used in the production of low-cost, large-area electronics in applications such as liquid crystal displays and of large-area, low-cost, thin-film solar cells. Such semiconductor grades of silicon are either slightly less pure or polycrystalline rather than monocrystalline, and are produced in comparable quatities as the monocrystalline silicon: 75,000 to 150,000 metric tons per year. The market for the lesser grade is growing more quickly than for monocrystalline silicon. By 2013, polycrystalline silicon production, used mostly in solar cells, was projected to reach 200,000 metric tons per year, while monocrystalline semiconductor grade silicon was expected to remain less than 50,000 tons/year.[63]
Biological role
A diatom, enclosed in a silica cell wall
Although silicon is readily available in the form of silicates, very few organisms use it directly. Diatoms, radiolaria and siliceous sponges use biogenic silica as a structural material for skeletons. In more advanced plants, the silica phytoliths (opal phytoliths) are rigid microscopic bodies occurring in the cell; some plants, for example rice, need silicon for their growth.[70][71][72] There is some evidence that silicon is important to nail, hair, bone and skin health in humans,[73] for example in studies that show that premenopausal women with higher dietary silicon intake have higher bone density, and that silicon supplementation can increase bone volume and density in patients with osteoporosis.[74] Silicon is needed for synthesis of elastin and collagen, of which the aorta contains the greatest quantity in the human body[75] and has been considered an essential element;[76] nevertheless, it is difficult to prove its essentiality, because silicon is very common and hence deficiency symptoms are difficult to reproduce.[77]
Silicon is currently under consideration for elevation to the status of a "plant beneficial substance by the Association of American Plant Food Control Officials (AAPFCO)."[78][79] Silicon has been shown to improve plant cell wall strength and structural integrity in some plants.[80]