Suspensions of gold nanoparticles of various sizes. The size difference causes the difference in colors.
Colloidal gold is a sol or colloidal suspension of nanoparticles of gold in a fluid, usually water. The colloid is usually either an intense red colour (for spherical particles less than 100 nm) or blue/purple (for larger spherical particles or nanorods).
Due to their optical,
electronic, and molecular-recognition properties, gold nanoparticles
are the subject of substantial research, with many potential or promised
applications in a wide variety of areas, including electron microscopy, electronics, nanotechnology, materials science, and biomedicine.
The properties of colloidal gold nanoparticles, and thus their
potential applications, depend strongly upon their size and shape. For example, rodlike particles have both transverse and longitudinal absorption peak, and anisotropy of the shape affects their self-assembly.
History
This cranberry glass bowl was made by adding a gold salt (probably gold chloride) to molten glass.
Used since ancient times as a method of staining glass colloidal gold was used in the 4th-century Lycurgus Cup, which changes color depending on the location of light source.
During the Middle Ages, soluble gold, a solution containing gold salt, had a reputation for its curative property for various diseases. In 1618, Francis Anthony, a philosopher and member of the medical profession, published a book called Panacea Aurea, sive tractatus duo de ipsius Auro Potabili (Latin: gold potion, or two treatments of potable
gold). The book introduces information on the formation of colloidal
gold and its medical uses. About half a century later, English botanist Nicholas Culpepper published book in 1656, Treatise of Aurum Potabile, solely discussing the medical uses of colloidal gold.
In 1676, Johann Kunckel, a German chemist, published a book on the manufacture of stained glass. In his book Valuable Observations or Remarks About the Fixed and Volatile Salts-Auro and Argento Potabile, Spiritu Mundi and the Like,
Kunckel assumed that the pink color of Aurum Potabile came from small
particles of metallic gold, not visible to human eyes. In 1842, John Herschel
invented a photographic process called chrysotype (from the Greek
χρῡσός meaning "gold") that used colloidal gold to record images on
paper.
Modern scientific evaluation of colloidal gold did not begin until Michael Faraday's work in the 1850s. In 1856, in a basement laboratory of Royal Institution, Faraday accidentally created a ruby red solution while mounting pieces of gold leaf onto microscope slides.
Since he was already interested in the properties of light and matter,
Faraday further investigated the optical properties of the colloidal
gold. He prepared the first pure sample of colloidal gold, which he
called 'activated gold', in 1857. He used phosphorus to reduce
a solution of gold chloride. The colloidal gold Faraday made 150 years
ago is still optically active. For a long time, the composition of the
'ruby' gold was unclear. Several chemists suspected it to be a gold tin compound, due to its preparation. Faraday recognized that the color was actually due to the miniature size of the gold particles. He noted the light scattering properties of suspended gold microparticles, which is now called Faraday-Tyndall effect.
In 1898, Richard Adolf Zsigmondy prepared the first colloidal gold in diluted solution. Apart from Zsigmondy, Theodor Svedberg, who invented ultracentrifugation, and Gustav Mie, who provided the theory for scattering and absorption by spherical particles, were also interested in the synthesis and properties of colloidal gold.
With advances in various analytical technologies in the 20th
century, studies on gold nanoparticles has accelerated. Advanced
microscopy methods, such as atomic force microscopy and electron microscopy,
have contributed the most to nanoparticle research. Due to their
comparably easy synthesis and high stability, various gold particles
have been studied for their practical uses. Different types of gold
nanoparticle are already used in many industries, such as medicine and
electronics. For example, several FDA-approved nanoparticles are currently used in drug delivery.
Physical properties
Optical
The variation of scattering cross section of 100 nm-radius gold nanoparticle vs. the wavelength
Colloidal gold has been used by artists for centuries because of the
nanoparticle’s interactions with visible light. Gold nanoparticles
absorb and scatter light
resulting in colours ranging from vibrant reds to blues to black and
finally to clear and colorless, depending on particle size, shape, local
refractive index, and aggregation state. These colors occur because of a
phenomenon called localized surface plasmon resonance (LSPR), in which conduction electrons on the surface of the nanoparticle oscillate in resonance with incident light.
Effect of size
As a general rule, the wavelength of light absorbed increases as a function of increasing nano particle size. For example, pseudo-spherical gold nanoparticles with diameters ~ 30 nm have a peak LSPR absorption at ~530 nm.
Effect of local refractive index
Changes
in the apparent color of a gold nanoparticle solution can also be
caused by the environment in which the colloidal gold is suspended
The optical properties of gold nanoparticles depends on the refractive
index near the nanoparticle surface, therefore both the molecules
directly attached to the nanoparticle surface (i.e. nanoparticle
ligands) and/or the nanoparticle solvent both may influence observed optical features. As the refractive index near the gold surface increases, the NP LSPR will shift to longer wavelengths In addition to solvent environment, the extinction peak can be tuned by coating the nanoparticles with non-conducting shells such as silica, bio molecules, or aluminium oxide.
Effect of aggregation
When
gold nano particles aggregate, the optical properties of the particle
change, because the effective particle size, shape, and dielectric environment all change.
Medical research
Electron microscopy
Colloidal gold and various derivatives have long been among the most widely used labels for antigens in biological electron microscopy. Colloidal gold particles can be attached to many traditional biological probes such as antibodies, lectins, superantigens, glycans, nucleic acids,
and receptors. Particles of different sizes are easily distinguishable
in electron micrographs, allowing simultaneous multiple-labelling
experiments.
In addition to biological probes, gold nanoparticles can be
transferred to various mineral substrates, such as mica, single crystal
silicon, and atomically flat gold(III), to be observed under atomic
force microscopy (AFM).
Drug delivery system
Gold
nanoparticles can be used to optimize the biodistribution of drugs to
diseased organs, tissues or cells, in order to improve and target drug
delivery.
Nanoparticle-mediated drug delivery is feasible only if the drug
distribution is otherwise inadequate. These cases include drug targeting
of unstable (proteins, siRNA, DNA),
delivery to the difficult sites (brain, retina, tumors, intracellular
organelles) and drugs with serious side effects (e.g. anti-cancer
agents). The performance of the nanoparticles depends on the size and
surface functionalities in the particles. Also, the drug release and
particle disintegration can vary depending on the system (e.g.
biodegradable polymers sensitive to pH). An optimal nanodrug delivery
system ensures that the active drug is available at the site of action
for the correct time and duration, and their concentration should be
above the minimal effective concentration (MEC) and below the minimal
toxic concentration (MTC).
Gold nanoparticles are being investigated as carriers for drugs such as Paclitaxel. The administration of hydrophobic drugs require molecular encapsulation and it is found that nanosized particles are particularly efficient in evading the reticuloendothelial system.
Tumor detection
In cancer research, colloidal gold can be used to target tumors and provide detection using SERS (surface enhanced Raman spectroscopy) in vivo.
These gold nanoparticles are surrounded with Raman reporters, which
provide light emission that is over 200 times brighter than quantum dots. It was found that the Raman reporters were stabilized when the nanoparticles were encapsulated with a thiol-modified polyethylene glycol coat. This allows for compatibility and circulation in vivo. To specifically target tumor cells, the polyethylenegylated gold particles are conjugated with an antibody (or an antibody fragment such as scFv), against, e.g. epidermal growth factor receptor,
which is sometimes overexpressed in cells of certain cancer types.
Using SERS, these pegylated gold nanoparticles can then detect the
location of the tumor.
Gold nanoparticles accumulate in tumors, due to the leakiness of
tumor vasculature, and can be used as contrast agents for enhanced
imaging in a time-resolved optical tomography system using short-pulse
lasers for skin cancer detection in mouse model. It is found that
intravenously administrated spherical gold nanoparticles broadened the
temporal profile of reflected optical signals and enhanced the contrast
between surrounding normal tissue and tumors.

Tumor
targeting via multifunctional nanocarriers. Cancer cells reduce
adhesion to neighboring cells and migrate into the vasculature-rich
stroma. Once at the vasculature, cells can freely enter the bloodstream.
Once the tumor is directly connected to the main blood circulation
system, multifunctional nanocarriers can interact directly with cancer
cells and effectively target tumors.
Gene therapy
Gold
nanoparticles have shown potential as intracellular delivery vehicles
for siRNA oligonucleotides with maximal therapeutic impact.

Multifunctional
siRNA-gold nanoparticles with several biomolecules: PEG, cell
penetration and cell adhesion peptides and siRNA. Two different
approaches were employed to conjugate the siRNA to the gold
nanoparticle: (1) Covalent approach: use of thiolated siRNA for gold-thiol binding to the nanoparticle; (2) Ionic approach: interaction of the negatively charged siRNA to the modified surface of the AuNP through ionic interactions.
Gold nanoparticles show potential as intracellular delivery vehicles for antisense oligonucleotides (ssDNA,dsDNA) by providing protection against intracellular nucleases and ease of functionalization for selective targeting.
Photothermal agents
Gold nanorods are being investigated as photothermal agents for in-vivo applications. Gold nanorods
are rod-shaped gold nanoparticles whose aspect ratios tune the surface
plasmon resonance (SPR) band from the visible to near-infrared
wavelength. The total extinction of light at the SPR is made up of both
absorption and scattering. For the smaller axial diameter nanorods
(~10 nm), absorption dominates, whereas for the larger axial diameter
nanorods (>35 nm) scattering can dominate. As a consequence, for
in-vivo studies, small diameter gold nanorods are being used as
photothermal converters of near-infrared light due to their high
absorption cross-sections.
Since near-infrared light transmits readily through human skin and
tissue, these nanorods can be used as ablation components for cancer,
and other targets. When coated with polymers, gold nanorods have been
observed to circulate in-vivo with half-lives longer than 6 hours,
bodily residence times around 72 hours, and little to no uptake in any
internal organs except the liver.
Despite the unquestionable success of gold nanorods as photothermal agents in preclinical research, they have yet to obtain the approval for clinical use because the size is above the renal excretion threshold. In 2019, the first NIR-absorbing plasmonic ultrasmall-in-nano architecture has been reported, and jointly combine: (i) a suitable photothermal conversion for hyperthermia treatments, (ii) the possibility of multiple photothermal treatments and (iii) renal excretion of the building blocks after the therapeutic action.
Radiotherapy dose enhancer
Considerable
interest has been shown in the use of gold and other
heavy-atom-containing nanoparticles to enhance the dose delivered to
tumors.
Since the gold nanoparticles are taken up by the tumors more than the
nearby healthy tissue, the dose is selectively enhanced. The biological
effectiveness of this type of therapy seems to be due to the local
deposition of the radiation dose near the nanoparticles. This mechanism is the same as occurs in heavy ion therapy.
Detection of toxic gas
Researchers have developed simple inexpensive methods for on-site detection of hydrogen sulfide H
2S present in air based on the antiaggregation of gold nanoparticles (AuNPs). Dissolving H
2S into a weak alkaline buffer solution leads to the formation of HS-, which can stabilize AuNPs and ensure they maintain their red color allowing for visual detection of toxic levels of H
2S.
2S present in air based on the antiaggregation of gold nanoparticles (AuNPs). Dissolving H
2S into a weak alkaline buffer solution leads to the formation of HS-, which can stabilize AuNPs and ensure they maintain their red color allowing for visual detection of toxic levels of H
2S.
Gold nanoparticle based biosensor
Gold nanoparticles are incorporated into biosensors to enhance its stability, sensitivity, and selectivity.
Nanoparticle properties such as small size, high surface-to-volume
ratio, and high surface energy allow immobilization of large range of
biomolecules. Gold nanoparticle, in particular, could also act as
"electron wire" to transport electrons and its amplification effect on
electromagnetic light allows it to function as signal amplifiers. Main types of gold nanoparticle based biosensors are optical and electrochemical biosensor.
Optical biosensor
Gold nanoparticle-based (Au-NP) biosensor for Glutathione (GSH). The AuNPs are functionalised
with a chemical group that binds to GSH and makes the NPs partially
collapse, and thus change colour. The exact amount of GSH can be derived
via UV-vis spectroscopy through a calibration curve.
Gold nanoparticles improve the sensitivity of optical sensor by
response to the change in local refractive index. The angle of the
incidence light for surface plasmon resonance, an interaction between
light wave and conducting electrons in metal, changes when other
substances are bounded to the metal surface. Because gold is very sensitive to its surroundings' dielectric constant,
binding of an analyte would significantly shift gold nanoparticle's SPR
and therefore allow more sensitive detection. Gold nanoparticle could
also amplify the SPR signal.
When the plasmon wave pass through the gold nanoparticle, the charge
density in the wave and the electron I the gold interacted and resulted
in higher energy response, so called electron coupling.
Since the analyte and bio-receptor now bind to the gold, it increases
the apparent mass of the analyte and therefore amplified the signal.
These properties had been used to build DNA sensor with 1000-fold sensitive than without the Au NP.
Humidity senor was also built by altering the atom interspacing between
molecules with humidity change, the interspacing change would also
result in a change of the Au NP's LSPR.
Electrochemical biosensor
Electrochemical
sensor convert biological information into electrical signals that
could be detected. The conductivity and biocompatibility of Au NP allow
it to act as "electron wire". It transfers electron between the electrode and the active site of the enzyme.
It could be accomplished in two ways: attach the Au NP to either the
enzyme or the electrode. GNP-glucose oxidase monolayer electrode was
constructed use these two methods.
The Au NP allowed more freedom in the enzyme's orientation and
therefore more sensitive and stable detection. Au NP also acts as
immobilization platform for the enzyme. Most biomolecules denatures or
lose its activity when interacted with the electrode.
The biocompatibility and high surface energy of Au allow it to bind to a
large amount of protein without altering its activity and results in a
more sensitive sensor. Moreover, Au NP also catalyzes biological reactions. Gold nanoparticle under 2 nm has shown catalytic activity to the oxidation of styrene.
Immunological biosensor
Gold nanoparticles have been coated with peptides and glycans for use in immunological detection methods. The possibility to use glyconanoparticles in ELISA
was unexpected, but the method seems to have a high sensitivity and
thus offers potential for development of specific assays for diagnostic identification of antibodies in patient sera
Thin films
Gold nanoparticles capped with organic ligands, such as alkanethiol molecules, can self-assemble into large monolayers (>cm).
The particles are first prepared in organic solvent, such as chloroform
or toluene, and are then spread into monolayers either on a liquid
surface or on a solid substrate. Such interfacial thin films of
nanoparticles have close relationship with Langmuir-Blodgett monolayers made from surfactants.
The mechanical properties of nanoparticle monolayers have been
studied extensively. For 5 nm spheres capped with dodecanethiol, the
Young's modulus of the monolayer is on the order of GPa. The mechanics of the membranes are guided by strong interactions between ligand shells on adjacent particles. Upon fracture, the films crack perpendicular to the direction of strain at a fracture stress of 11 2.6 MPa, comparable to that of cross-linked polymer films. Free-standing nanoparticle membranes exhibit bending rigidity on the order of 10
eV, higher than what is predicted in theory for continuum plates of the
same thickness, due to nonlocal microstructural constraints such as
nonlocal coupling of particle rotational degrees of freedom.
On the other hand, resistance to bending is found to be greatly reduced
in nanoparticle monolayers that are supported at the air/water
interface, possibly due to screening of ligand interactions in a wet
environment.
Surface chemistry
In many different types of colloidal gold syntheses, the interface of the nanoparticles can display widely different character – ranging from an interface similar to a self-assembled monolayer to a disordered boundary with no repeating patterns. Beyond the Au-Ligand interface, conjugation of the interfacial ligands with various functional moieties (from small organic molecules to polymers to DNA to RNA) afford colloidal gold much of its vast functionality.
Ligand exchange/functionalization
After
initial nanoparticle synthesis, colloidal gold ligands are often
exchanged with new ligands designed for specific applications. For
example, Au NPs produced via the Turkevich-style (or Citrate Reduction)
method are readily reacted via ligand exchange reactions, due to the
relatively weak binding between the carboxyl groups and the surfaces of
the NPs. This ligand exchange can produce conjugation with a number of biomolecules from DNA to RNA to proteins to polymers (such as PEG) to increase biocompatibility and functionality. For example, ligands have been shown to enhance catalytic activity by mediating interactions between adsorbates and the active gold surfaces for specific oxygenation reactions. Ligand exchange can also be used to promote phase transfer of the colloidal particles.
Ligand exchange is also possible with alkane thiol-arrested NPs
produced from the Brust-type synthesis method, although higher
temperatures are needed to promote the rate of the ligand detachment.
An alternative method for further functionalization is achieved through
the conjugation of the ligands with other molecules, though this method
can cause the colloidal stability of the Au NPs to breakdown.
Ligand removal
In
many cases, as in various high-temperature catalytic applications of
Au, the removal of the capping ligands produces more desirable
physicochemical properties.
The removal of ligands from colloidal gold while maintaining a
relatively constant number of Au atoms per Au NP can be difficult due to
the tendency for these bare clusters to aggregate. The removal of
ligands is partially achievable by simply washing away all excess
capping ligands, though this method is ineffective in removing all
capping ligand. More often ligand removal achieved under high
temperature or light ablation followed by washing. Alternatively, the ligands can be electrochemically etched off.
Surface structure and chemical environment
The
precise structure of the ligands on the surface of colloidal gold NPs
impact the properties of the colloidal gold particles. Binding
conformations and surface packing of the capping ligands at the surface
of the colloidal gold NPs tend to differ greatly from bulk surface model
adsorption, largely due to the high curvature observed at the
nanoparticle surfaces.
Thiolate-gold interfaces at the nanoscale have been well-studied and
the thiolate ligands are observed to pull Au atoms off of the surface of
the particles to for “staple” motifs that have significant Thiyl-Au(0)
character.
The citrate-gold surface, on the other hand, is relatively less-studied
due to the vast number of binding conformations of the citrate to the
curved gold surfaces. A study performed in 2014 identified that the
most-preferred binding of the citrate involves two carboxylic acids and
the hydroxyl group of the citrate binds three surface metal atoms.
Health and safety
As gold nanoparticles (AuNPs) are further investigated for targeted
drug delivery in humans, their toxicity needs to be considered. For the
most part, it is suggested that AuNPs are biocompatible,
but the concentrations at which they become toxic needs to be
determined, and if those concentrations fall within the range of used
concentrations. Toxicity can be tested in vitro and in vivo. In vitro
toxicity results can vary depending on the type of the cellular growth
media with different protein compositions, the method used to determine
cellular toxicity (cell health, cell stress, how many cells are taken
into a cell), and the capping ligands in solution. In vivo
assessments can determine the general health of an organism (abnormal
behavior, weight loss, average life span) as well as tissue specific
toxicology (kidney, liver, blood) and inflammation and oxidative
responses. In vitro experiments are more popular than in vivo experiments because in vitro experiments are more simplistic to perform than in vivo experiments.
Toxicity and hazards in synthesis
While AuNPs themselves appear to have low or negligible toxicity,
and the literature shows that the toxicity has much more to do with the
ligands rather than the particles themselves, the synthesis of them
involves chemicals that are hazardous. Sodium borohydride, a harsh reagent, is used to reduce the gold ions to gold metal. The gold ions usually come from chloroauric acid, a potent acid.
Because of the high toxicity and hazard of reagents used to synthesize
AuNPs, the need for more “green” methods of synthesis arose.
Toxicity due to capping ligands
Some
of the capping ligands associated with AuNPs can be toxic while others
are nontoxic. In gold nanorods (AuNRs), it has been shown that a strong
cytotoxicity was associated with CTAB-stabilized AuNRs at low concentration, but it is thought that free CTAB was the culprit in toxicity.
Modifications that overcoat these AuNRs reduces this toxicity in human
colon cancer cells (HT-29) by preventing CTAB molecules from desorbing
from the AuNRs back into the solution.
Ligand toxicity can also be seen in AuNPs. Compared to the 90% toxicity
of HAuCl4 at the same concentration, AuNPs with carboxylate termini were
shown to be non-toxic. Large AuNPs conjugated with biotin, cysteine, citrate, and glucose were not toxic in human leukemia cells (K562) for concentrations up to 0.25 M.
Also, citrate-capped gold nanospheres (AuNSs) have been proven to be
compatible with human blood and did not cause platelet aggregation or an
immune response.
However, citrate-capped gold nanoparticles sizes 8-37 nm were found to
be lethally toxic for mice, causing shorter lifespans, severe sickness,
loss of appetite and weight, hair discoloration, and damage to the
liver, spleen, and lungs; gold nanoparticles accumulated in the spleen
and liver after traveling a section of the immune system.
There are mixed-views for polyethylene glycol
(PEG)-modified AuNPs. These AuNPs were found to be toxic in mouse liver
by injection, causing cell death and minor inflammation. However, AuNPs conjugated with PEG copolymers showed negligible toxicity towards human colon cells (Caco-2).
AuNP toxicity also depends on the overall charge of the ligands. In
certain doses, AuNSs that have positively-charged ligands are toxic in
monkey kidney cells (Cos-1), human red blood cells, and E. coli because
of the AuNSs interaction with the negatively-charged cell membrane;
AuNSs with negatively-charged ligands have been found to be nontoxic in
these species.
In addition to the previously mentioned in vivo and in vitro
experiments, other similar experiments have been performed.
Alkylthiolate-AuNPs with trimethlyammonium ligand termini mediate the translocation of DNA across mammalian cell membranes in vitro at a high level, which is detrimental to these cells. Corneal haze in rabbits have been healed in vivo
by using polyethylemnimine-capped gold nanoparticles that were
transfected with a gene that promotes wound healing and inhibits corneal
fibrosis.
Toxicity due to size of nanoparticles
Toxicity
in certain systems can also be dependent on the size of the
nanoparticle. AuNSs size 1.4 nm were found to be toxic in human skin
cancer cells (SK-Mel-28), human cervical cancer cells (HeLa), mouse fibroblast cells (L929), and mouse macrophages (J774A.1), while 0.8, 1.2, and 1.8 nm sized AuNSs were less toxic by a six-fold amount and 15 nm AuNSs were nontoxic. There is some evidence for AuNP buildup after injection in in vivo studies, but this is very size dependent. 1.8 nm AuNPs were found to be almost totally trapped in the lungs of rats. Different sized AuNPs were found to buildup in the blood, brain, stomach, pancreas, kidneys, liver, and spleen.
Biosafety
and biokinetics investigations on biodegradable ultrasmall-in-nano
architectures have demonstrated that gold nanoparticles are able to
avoid metal accumulation in organisms through escaping by the renal
pathway.
Synthesis
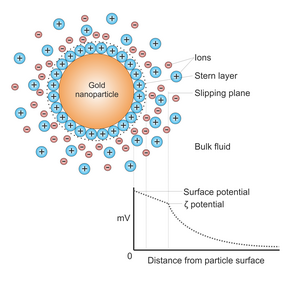
Potential difference as a function of distance from particle surface.
Generally, gold nanoparticles are produced in a liquid ("liquid chemical methods") by reduction of chloroauric acid (H[AuCl4]).
To prevent the particles from aggregating, stabilizing agents are
added. Citrate acts both as the reducing agent and colloidal
stabilizer.
They can be functionalized with various organic ligands to create organic-inorganic hybrids with advanced functionality.
Turkevich method
This simple method was pioneered by J. Turkevich et al. in 1951 and refined by G. Frens in the 1970s. It produces modestly monodisperse
spherical gold nanoparticles of around 10–20 nm in diameter. Larger
particles can be produced, but at the cost of monodispersity and shape.
In this method, hot chloroauric acid is treated with sodium citrate solution, producing colloidal gold. The Turkevich reaction proceeds via formation of transient gold nanowires. These gold nanowires are responsible for the dark appearance of the reaction solution before it turns ruby-red.
Capping agents
A
capping agent is used during nanoparticle synthesis to inhibit particle
growth and aggregation. The chemical blocks or reduces reactivity at
the periphery of the particle—a good capping agent has a high affinity
for the new nuclei. Citrate ions or tannic acid function both as a reducing agent and a capping agent. Less sodium citrate results in larger particles.
Brust-Schiffrin method
This method was discovered by Brust and Schiffrin in the early 1990s, and can be used to produce gold nanoparticles in organic liquids that are normally not miscible with water (like toluene). It involves the reaction of a chlorauric acid solution with tetraoctylammonium bromide (TOAB) solution in toluene and sodium borohydride as an anti-coagulant and a reducing agent, respectively.
Here, the gold nanoparticles will be around 5–6 nm. NaBH4 is the reducing agent, and TOAB is both the phase transfer catalyst and the stabilizing agent.
TOAB does not bind to the gold nanoparticles particularly
strongly, so the solution will aggregate gradually over the course of
approximately two weeks. To prevent this, one can add a stronger binding
agent, like a thiol (in particular, alkanethiols), which will bind to gold, producing a near-permanent solution.
Alkanethiol protected gold nanoparticles can be precipitated and then
redissolved. Thiols are better binding agents because there is a strong
affinity for the gold-sulfur bonds that form when the two substances
react with each other. Tetra-dodecanthiol is a commonly used strong binding agent to synthesize smaller particles.
Some of the phase transfer agent may remain bound to the purified nanoparticles, this may affect physical properties such as solubility. In order to remove as much of this agent as possible, the nanoparticles must be further purified by soxhlet extraction.
Perrault method
This approach, discovered by Perrault and Chan in 2009, uses hydroquinone to reduce HAuCl4
in an aqueous solution that contains 15 nm gold nanoparticle seeds.
This seed-based method of synthesis is similar to that used in
photographic film development, in which silver grains within the film
grow through addition of reduced silver onto their surface. Likewise,
gold nanoparticles can act in conjunction with hydroquinone to catalyze
reduction of ionic gold onto their surface. The presence of a stabilizer
such as citrate results in controlled deposition of gold atoms onto the
particles, and growth. Typically, the nanoparticle seeds are produced
using the citrate method. The hydroquinone method complements that of
Frens,
as it extends the range of monodispersed spherical particle sizes that
can be produced. Whereas the Frens method is ideal for particles of
12–20 nm, the hydroquinone method can produce particles of at least
30–300 nm.
Martin method
This simple method, discovered by Martin and Eah in 2010,
generates nearly monodisperse "naked" gold nanoparticles in water.
Precisely controlling the reduction stoichiometry by adjusting the ratio
of NaBH4-NaOH ions to HAuCl4-HCl ions within the
"sweet zone," along with heating, enables reproducible diameter tuning
between 3–6 nm. The aqueous particles are colloidally stable due to
their high charge from the excess ions in solution. These particles can
be coated with various hydrophilic functionalities, or mixed with
hydrophobic ligands for applications in non-polar solvents. In non-polar
solvents the nanoparticles remain highly charged, and self-assemble on
liquid droplets to form 2D monolayer films of monodisperse
nanoparticles.
Nanotech studies
Bacillus licheniformis can be used in synthesis of gold nanocubes with sizes between 10 and 100 nanometres.
Gold nanoparticles are usually synthesized at high temperatures in
organic solvents or using toxic reagents. The bacteria produce them in
much milder conditions.
For
particles larger than 30 nm, control of particle size with a low
polydispersity of spherical gold nanoparticles remains challenging. In
order to provide maximum control on the NP structure, Navarro and
co-workers used a modified Turkevitch-Frens procedure using sodium
acetylacetonate Na(acac) as the reducing agent and sodium citrate as the
stabilizer.
Sonolysis
Another method for the experimental generation of gold particles is by sonolysis. The first method of this type was invented by Baigent and Müller.
This work pioneered the use of ultrasound to provide the energy for the
processes involved and allowed the creation of gold particles with a
diameter of under 10 nm. In another method using ultrasound, the
reaction of an aqueous solution of HAuCl4 with glucose, the reducing agents are hydroxyl radicals and sugar pyrolysis radicals
(forming at the interfacial region between the collapsing cavities and
the bulk water) and the morphology obtained is that of nanoribbons with
width 30–50 nm and length of several micrometers. These ribbons are very
flexible and can bend with angles larger than 90°. When glucose is
replaced by cyclodextrin
(a glucose oligomer), only spherical gold particles are obtained,
suggesting that glucose is essential in directing the morphology toward a
ribbon.
Block copolymer-mediated method
An
economical, environmentally benign and fast synthesis methodology for
gold nanoparticles using block copolymer has been developed by Sakai et
al.
In this synthesis methodology, block copolymer plays the dual role of a
reducing agent as well as a stabilizing agent. The formation of gold
nanoparticles comprises three main steps: reduction of gold salt ion by
block copolymers in the solution and formation of gold clusters,
adsorption of block copolymers on gold clusters and further reduction of
gold salt ions on the surfaces of these gold clusters for the growth of
gold particles in steps, and finally its stabilization by block
copolymers. But this method usually has a limited-yield (nanoparticle
concentration), which does not increase with the increase in the gold
salt concentration. Ray et al. improved this synthesis method by enhancing the nanoparticle yield by manyfold at ambient temperature.