From Wikipedia, the free encyclopedia
Beckman DU640 UV/Vis spectrophotometer
UV spectroscopy or UV–visible spectrophotometry (UV–Vis or UV/Vis) refers to absorption spectroscopy or reflectance spectroscopy in part of the ultraviolet and the full, adjacent visible regions of the electromagnetic spectrum.
Being relatively inexpensive and easily implemented, this methodology
is widely used in diverse applied and fundamental applications. The
only requirement is that the sample absorb in the UV-vis region, i.e. be
a chromophore. Absorption spectroscopy is complementary to fluorescence spectroscopy.
Parameters of interest, besides the wavelength of measurement, are
absorbance (A) or Transmittance (%T) or Reflectance (%R), and its change
with time.
Optical transitions
Most
molecules and ions absorb energy in the ultraviolet or visible range,
i.e., they are chromophores. The absorbed photon excites an electron in
the chromophore to higher energy molecular orbitals, giving rise to an excited state.
For organic chromophores, four possible types of transitions are
assumed: π–π*, n–π*, σ–σ*, and n–σ*. Transition metal complexes are
often colored (i.e., absorb visible light) owing to the presence of
multiple electronic states associated with incompletely filled d
orbitals.
Applications
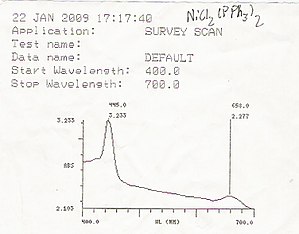
An example of a UV/Vis readout
UV/Vis spectroscopy is routinely used in analytical chemistry for the quantitative determination of diverse analytes or sample, such as transition metal ions, highly conjugated organic compounds,
and biological macromolecules. Spectroscopic analysis is commonly
carried out in solutions but solids and gases may also be studied.
- Organic compounds, especially those with a high degree of conjugation, also absorb light in the UV or visible regions of the electromagnetic spectrum. The solvents for these determinations are often water for water-soluble compounds, or ethanol
for organic-soluble compounds. (Organic solvents may have significant
UV absorption; not all solvents are suitable for use in UV spectroscopy.
Ethanol absorbs very weakly at most wavelengths.) Solvent polarity and
pH can affect the absorption spectrum of an organic compound. Tyrosine,
for example, increases in absorption maxima and molar extinction
coefficient when pH increases from 6 to 13 or when solvent polarity
decreases.
- While charge transfer complexes also give rise to colours, the colours are often too intense to be used for quantitative measurement.
The Beer–Lambert law
states that the absorbance of a solution is directly proportional to
the concentration of the absorbing species in the solution and the path
length.
Thus, for a fixed path length, UV/Vis spectroscopy can be used to
determine the concentration of the absorber in a solution. It is
necessary to know how quickly the absorbance changes with concentration.
This can be taken from references (tables of molar extinction coefficients), or more accurately, determined from a calibration curve.
A UV/Vis spectrophotometer may be used as a detector for HPLC.
The presence of an analyte gives a response assumed to be proportional
to the concentration. For accurate results, the instrument's response to
the analyte in the unknown should be compared with the response to a
standard; this is very similar to the use of calibration curves. The
response (e.g., peak height) for a particular concentration is known as
the response factor.
The wavelengths of absorption peaks can be correlated with the
types of bonds in a given molecule and are valuable in determining the
functional groups within a molecule. The Woodward–Fieser rules, for instance, are a set of empirical observations used to predict λmax, the wavelength of the most intense UV/Vis absorption, for conjugated organic compounds such as dienes and ketones.
The spectrum alone is not, however, a specific test for any given
sample. The nature of the solvent, the pH of the solution, temperature,
high electrolyte concentrations, and the presence of interfering
substances can influence the absorption spectrum. Experimental
variations such as the slit width (effective bandwidth) of the
spectrophotometer will also alter the spectrum. To apply UV/Vis
spectroscopy to analysis, these variables must be controlled or
accounted for in order to identify the substances present.
The method is most often used in a quantitative way to determine concentrations of an absorbing species in solution, using the Beer–Lambert law:
 ,
,
where A is the measured absorbance (in Absorbance Units (AU)),  is the intensity of the incident light at a given wavelength,
is the intensity of the incident light at a given wavelength,  is the transmitted intensity, L the path length through the sample, and c the concentration of the absorbing species. For each species and wavelength, ε is a constant known as the molar absorptivity
or extinction coefficient. This constant is a fundamental molecular
property in a given solvent, at a particular temperature and pressure,
and has units of
is the transmitted intensity, L the path length through the sample, and c the concentration of the absorbing species. For each species and wavelength, ε is a constant known as the molar absorptivity
or extinction coefficient. This constant is a fundamental molecular
property in a given solvent, at a particular temperature and pressure,
and has units of  .
.
The absorbance and extinction ε are sometimes defined in terms of the natural logarithm instead of the base-10 logarithm.
The Beer–Lambert Law is useful for characterizing many compounds
but does not hold as a universal relationship for the concentration and
absorption of all substances. A 2nd order polynomial relationship
between absorption and concentration is sometimes encountered for very
large, complex molecules such as organic dyes (Xylenol Orange or Neutral Red, for example).
UV–Vis spectroscopy is also used in the semiconductor industry to
measure the thickness and optical properties of thin films on a wafer.
UV–Vis spectrometers are used to measure the reflectance of light, and
can be analyzed via the Forouhi–Bloomer dispersion equations to determine the index of refraction ( ) and the extinction coefficient (
) and the extinction coefficient ( ) of a given film across the measured spectral range.
) of a given film across the measured spectral range.
Practical considerations
The
Beer–Lambert law has implicit assumptions that must be met
experimentally for it to apply; otherwise there is a possibility of
deviations from the law.
For instance, the chemical makeup and physical environment of the
sample can alter its extinction coefficient. The chemical and physical
conditions of a test sample therefore must match reference measurements
for conclusions to be valid. Worldwide, pharmacopoeias such as the
American (USP) and European (Ph. Eur.) pharmacopeias demand that
spectrophotometers perform according to strict regulatory requirements
encompassing factors such as stray light and wavelength accuracy.
Spectral bandwidth
It is important to have a monochromatic source of radiation for the light incident on the sample cell.
Monochromaticity is measured as the width of the "triangle" formed by
the intensity spike, at one half of the peak intensity. A given
spectrometer has a spectral bandwidth that characterizes how monochromatic the incident light is. If this bandwidth is comparable to (or more than) the width
of the absorption line, then the measured extinction coefficient will
be mistaken. In reference measurements, the instrument bandwidth
(bandwidth of the incident light) is kept below the width of the
spectral lines. When a test material is being measured, the bandwidth of
the incident light should also be sufficiently narrow. Reducing the
spectral bandwidth reduces the energy passed to the detector and will,
therefore, require a longer measurement time to achieve the same signal
to noise ratio.
Wavelength error
In
liquids, the extinction coefficient usually changes slowly with
wavelength. A peak of the absorbance curve (a wavelength where the
absorbance reaches a maximum) is where the rate of change in absorbance
with wavelength is smallest.
Measurements are usually made at a peak to minimize errors produced by
errors in wavelength in the instrument, that is errors due to having a
different extinction coefficient than assumed.
Stray light
Another important major factor is the purity of the light used. The most important factor affecting this is the stray light level of the monochromator.
The detector used is broadband; it responds to all the light that
reaches it. If a significant amount of the light passed through the
sample contains wavelengths that have much lower extinction coefficients
than the nominal one, the instrument will report an incorrectly low
absorbance. Any instrument will reach a point where an increase in
sample concentration will not result in an increase in the reported
absorbance, because the detector is simply responding to the stray
light. In practice the concentration of the sample or the optical path
length must be adjusted to place the unknown absorbance within a range
that is valid for the instrument. Sometimes an empirical calibration
function is developed, using known concentrations of the sample, to
allow measurements into the region where the instrument is becoming
non-linear.
As a rough guide, an instrument with a single monochromator would
typically have a stray light level corresponding to about 3 Absorbance
Units (AU), which would make measurements above about 2 AU problematic. A
more complex instrument with a double monochromator
would have a stray light level corresponding to about 6 AU, which would
therefore allow measuring a much wider absorbance range.
Deviations from the Beer–Lambert law
At
sufficiently high concentrations, the absorption bands will saturate
and show absorption flattening. The absorption peak appears to flatten
because close to 100% of the light is already being absorbed. The
concentration at which this occurs depends on the particular compound
being measured. One test that can be used to test for this effect is to
vary the path length of the measurement. In the Beer–Lambert law,
varying concentration and path length has an equivalent effect—diluting a
solution by a factor of 10 has the same effect as shortening the path
length by a factor of 10. If cells of different path lengths are
available, testing if this relationship holds true is one way to judge
if absorption flattening is occurring.
Solutions that are not homogeneous can show deviations from the
Beer–Lambert law because of the phenomenon of absorption flattening.
This can happen, for instance, where the absorbing substance is located
within suspended particles.
The deviations will be most noticeable under conditions of low
concentration and high absorbance. The last reference describes a way to
correct for this deviation.
Some solutions, like copper(II)chloride in water, change visually
at a certain concentration because of changed conditions around the
coloured ion (the divalent copper ion). For copper(II)chloride it means a
shift from blue to green, which would mean that monochromatic measurements would deviate from the Beer–Lambert law.
Measurement uncertainty sources
The above factors contribute to the measurement uncertainty
of the results obtained with UV/Vis spectrophotometry. If UV/Vis
spectrophotometry is used in quantitative chemical analysis then the
results are additionally affected by uncertainty sources arising from
the nature of the compounds and/or solutions that are measured. These
include spectral interferences caused by absorption band overlap, fading
of the color of the absorbing species (caused by decomposition or
reaction) and possible composition mismatch between the sample and the
calibration solution.
Ultraviolet–visible spectrophotometer
The instrument used in ultraviolet–visible spectroscopy is called a UV/Vis spectrophotometer. It measures the intensity of light after passing through a sample ( ), and compares it to the intensity of light before it passes through the sample (
), and compares it to the intensity of light before it passes through the sample ( ). The ratio
). The ratio  is called the transmittance, and is usually expressed as a percentage (%T). The absorbance,
is called the transmittance, and is usually expressed as a percentage (%T). The absorbance,  , is based on the transmittance:
, is based on the transmittance:

The UV–visible spectrophotometer can also be configured to measure
reflectance. In this case, the spectrophotometer measures the intensity
of light reflected from a sample ( ), and compares it to the intensity of light reflected from a reference material (
), and compares it to the intensity of light reflected from a reference material ( ) (such as a white tile). The ratio
) (such as a white tile). The ratio  is called the reflectance, and is usually expressed as a percentage (%R).
is called the reflectance, and is usually expressed as a percentage (%R).
The basic parts of a spectrophotometer are a light source, a holder for the sample, a diffraction grating in a monochromator or a prism to separate the different wavelengths of light, and a detector. The radiation source is often a Tungsten filament (300–2500 nm), a deuterium arc lamp, which is continuous over the ultraviolet region (190–400 nm), Xenon arc lamp, which is continuous from 160 to 2,000 nm; or more recently, light emitting diodes (LED) for the visible wavelengths. The detector is typically a photomultiplier tube, a photodiode, a photodiode array or a charge-coupled device
(CCD). Single photodiode detectors and photomultiplier tubes are used
with scanning monochromators, which filter the light so that only light
of a single wavelength reaches the detector at one time. The scanning
monochromator moves the diffraction grating to "step-through" each
wavelength so that its intensity may be measured as a function of
wavelength. Fixed monochromators are used with CCDs and photodiode
arrays. As both of these devices consist of many detectors grouped into
one or two dimensional arrays, they are able to collect light of
different wavelengths on different pixels or groups of pixels
simultaneously.
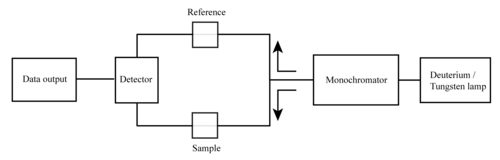
Simplified schematic of a double beam UV–visible spectrophotometer
A spectrophotometer can be either single beam or double beam. In a single beam instrument (such as the Spectronic 20), all of the light passes through the sample cell.  must be measured by removing the sample. This was the earliest design
and is still in common use in both teaching and industrial labs.
must be measured by removing the sample. This was the earliest design
and is still in common use in both teaching and industrial labs.
In a double-beam instrument, the light is split into two beams
before it reaches the sample. One beam is used as the reference; the
other beam passes through the sample. The reference beam intensity is
taken as 100% Transmission (or 0 Absorbance), and the measurement
displayed is the ratio of the two beam intensities. Some double-beam
instruments have two detectors (photodiodes), and the sample and
reference beam are measured at the same time. In other instruments, the
two beams pass through a beam chopper,
which blocks one beam at a time. The detector alternates between
measuring the sample beam and the reference beam in synchronism with the
chopper. There may also be one or more dark intervals in the chopper
cycle. In this case, the measured beam intensities may be corrected by
subtracting the intensity measured in the dark interval before the ratio
is taken.
In a single-beam instrument, the cuvette containing only a
solvent has to be measured first. Mettler Toledo developed a single beam
array spectrophotometer that allows fast and accurate measurements over
the UV/VIS range. The light source consists of a Xenon flash lamp for
the ultraviolet (UV) as well as for the visible (VIS) and near-infrared
wavelength regions covering a spectral range from 190 up to 1100 nm. The
lamp flashes are focused on a glass fiber which drives the beam of
light onto a cuvette containing the sample solution. The beam passes
through the sample and specific wavelengths are absorbed by the sample
components. The remaining light is collected after the cuvette by a
glass fiber and driven into a spectrograph. The spectrograph consists of
a diffraction grating that separates the light into the different
wavelengths, and a CCD sensor to record the data, respectively. The
whole spectrum is thus simultaneously measured, allowing for fast
recording.
Samples for UV/Vis spectrophotometry are most often liquids,
although the absorbance of gases and even of solids can also be
measured. Samples are typically placed in a transparent cell, known as a cuvette. Cuvettes are typically rectangular in shape, commonly with an internal width of 1 cm. (This width becomes the path length,  , in the Beer–Lambert law.) Test tubes
can also be used as cuvettes in some instruments. The type of sample
container used must allow radiation to pass over the spectral region of
interest. The most widely applicable cuvettes are made of high quality fused silica or quartz glass
because these are transparent throughout the UV, visible and near
infrared regions. Glass and plastic cuvettes are also common, although
glass and most plastics absorb in the UV, which limits their usefulness
to visible wavelengths.
, in the Beer–Lambert law.) Test tubes
can also be used as cuvettes in some instruments. The type of sample
container used must allow radiation to pass over the spectral region of
interest. The most widely applicable cuvettes are made of high quality fused silica or quartz glass
because these are transparent throughout the UV, visible and near
infrared regions. Glass and plastic cuvettes are also common, although
glass and most plastics absorb in the UV, which limits their usefulness
to visible wavelengths.
Specialized instruments have also been made. These include
attaching spectrophotometers to telescopes to measure the spectra of
astronomical features. UV–visible microspectrophotometers consist of a
UV–visible microscope integrated with a UV–visible spectrophotometer.
A complete spectrum of the absorption at all wavelengths of
interest can often be produced directly by a more sophisticated
spectrophotometer. In simpler instruments the absorption is determined
one wavelength at a time and then compiled into a spectrum by the
operator. By removing the concentration dependence, the extinction
coefficient (ε) can be determined as a function of wavelength.
Microspectrophotometry
UV–visible
spectroscopy of microscopic samples is done by integrating an optical
microscope with UV–visible optics, white light sources, a monochromator, and a sensitive detector such as a charge-coupled device (CCD) or photomultiplier
tube (PMT). As only a single optical path is available, these are
single beam instruments. Modern instruments are capable of measuring
UV–visible spectra in both reflectance and transmission of micron-scale
sampling areas. The advantages of using such instruments is that they
are able to measure microscopic samples but are also able to measure the
spectra of larger samples with high spatial resolution. As such, they
are used in the forensic laboratory to analyze the dyes and pigments in
individual textile fibers, microscopic paint chips and the color of glass fragments. They are also used in materials
science and biological research and for determining the energy content
of coal and petroleum source rock by measuring the vitrinite
reflectance. Microspectrophotometers are used in the semiconductor and
micro-optics industries for monitoring the thickness of thin films after
they have been deposited. In the semiconductor industry, they are used
because the critical dimensions of circuitry is microscopic. A typical
test of a semiconductor wafer would entail the acquisition of spectra
from many points on a patterned or unpatterned wafer. The thickness of
the deposited films may be calculated from the interference pattern
of the spectra. In addition, ultraviolet–visible spectrophotometry can
be used to determine the thickness, along with the refractive index and
extinction coefficient of thin films. A map of the film thickness across the entire wafer can then be generated and used for quality control purposes.
Additional applications
UV/Vis can be applied to characterize the rate of a chemical reaction.
Illustrative is the conversion of the yellow-orange and blue isomers of
mercury dithizonate. This method of analysis relies on the fact that
concentration is linearly proportional to concentration. In the same
approach allows determination of equilibria between chromophores.
From the spectrum of burning gases, it is possible to determine a
chemical composition of a fuel, temperature of gases, and air-fuel
ratio.