What constitutes a definition of fascism and fascist governments has been a complicated and highly disputed subject concerning the exact nature of fascism and its core tenets debated amongst historians, political scientists, and other scholars since Benito Mussolini first used the term in 1915. Historian Ian Kershaw once wrote that "trying to define 'fascism' is like trying to nail jelly to the wall". A significant number of scholars agree that a "fascist regime" is foremost an authoritarian form of government, although not all authoritarian regimes are fascist. Authoritarianism is thus a defining characteristic, but most scholars will say that more distinguishing traits are needed to make an authoritarian regime fascist. Similarly, fascism as an ideology is also hard to define. Originally, it referred to a totalitarian political movement linked with corporatism which existed in Italy from 1922 to 1943 under the leadership of Benito Mussolini. Many scholars use the word "fascism" without capitalization in a more general sense, to refer to an ideology (or group of ideologies) which was influential in many countries at many different times. For this purpose, they have sought to identify what Roger Griffin calls a "fascist minimum"—that is, the minimum conditions that a certain political movement must meet in order to be considered "fascist". Scholars have studied the apocalyptic and millenarian aspects of fascism. By encyclopediasEncyclopaedia BritannicaThe Encyclopaedia Britannica defines fascism as a "political ideology and mass movement that dominated many parts of central, southern, and eastern Europe between 1919 and 1945 and that also had adherents in western Europe, the United States, South Africa, Japan, Latin America, and the Middle East.", adding that "Although fascist parties and movements differed significantly from one another, they had many characteristics in common, including extreme militaristic nationalism, contempt for electoral democracy and political and cultural liberalism, a belief in natural social hierarchy and the rule of elites, and the desire to create a Volksgemeinschaft (German: "people's community"), in which individual interests would be subordinated to the good of the nation." Holocaust EncyclopediaThe Holocaust Encyclopedia defines fascism as "a far-right political philosophy, or theory of government, that emerged in the early twentieth century. Fascism prioritizes the nation over the individual, who exists to serve the nation." and as "an ultranationalist, authoritarian political philosophy. It combines elements of nationalism, militarism, economic self-sufficiency, and totalitarianism. It opposes communism, socialism, pluralism, individual rights and equality, and democratic government." By fascistsBenito MussoliniBenito Mussolini, who was the first to use the term for his political party in 1915, described fascism in The Doctrine of Fascism, published in 1932, as follows:
In a speech before the Chamber of Deputies on 26 May 1927, Mussolini said:
Francisco FrancoIn an interview with Henri Massis in 1938, Spanish Nationalist leader Francisco Franco described his movement in Spain as part of a wider trend and said about this trend:
By scholarsUmberto EcoIn his 1995 essay "Ur-Fascism", cultural theorist Umberto Eco lists fourteen general properties of fascist ideology. He argues that it is not possible to organise these into a coherent system, but that "it is enough that one of them be present to allow fascism to coagulate around it". He uses the term "ur-fascism" as a generic description of different historical forms of fascism. The fourteen properties are as follows:
Emilio GentileItalian historian of fascism Emilio Gentile described fascism in 1996 as the "sacralization of politics" through totalitarian methods and argued the following ten constituent elements:
A. James GregorA. James Gregor, co-founder of the International Association for the Advancement of Ethnology and Eugenics, a prominent group in the promotion of eugenics and segregation, affirmed that fascism was a "variant of Sorelian syndicalism" which also included components of neo-idealism and elitist socialism. Gregor stated that Stalinism and Fascist totalitarianism would have been impossible without the "transmogrified Marxism, that infilled both". According to Gregor:
Furthermore, he believed that post-Maoist China displays many fascist traits. He has denied that fascism is "right-wing extremism". Gregor's work has come under increasing criticism in the 21st century. In a review of his 2006 essay on Neofascist movements, Peter H. Merkl, of the University of California, Santa Barbara, accuses Gregor of ignoring modern work in favor of his own views, and attempting to force an out of date definition of fascism. Merkl writes:
Roger GriffinHistorian and political scientist Roger Griffin's definition of fascism focuses on the populist fascist rhetoric that argues for a "re-birth" of a conflated nation and ethnic people. According to Griffin,
Griffin writes that a broad scholarly consensus developed in English-speaking social sciences during the 1990s, around the following definition of fascism:
Griffin argues that the above definition can be condensed into one sentence: "Fascism is a political ideology whose mythic core in its various permutations is a palingenetic form of populist ultra-nationalism." The word "palingenetic" in this case refers to notions of national rebirth. Ian KershawIn his history of Europe in the first half of the 20th century, To Hell and Back, British historian Ian Kershaw, while noting the difficulties in defining fascism, found these common factors in the extreme Right-wing movements of the late 1920s and early 1930s, whether they called themselves "fascist" or not:
Other features Kershaw found to be important, and sometimes central to specific movements, but not present in all:
Kershaw argues that the difference between fascism and other forms of right-wing authoritarianism in the Interwar period is that the latter generally aimed "to conserve the existing social order", whereas fascism was "revolutionary", seeking to change society and obtain "total commitment" from the population. Kershaw writes about the essential appeal of fascism and the reasons for its success, where it was successful (primarily in Italy and Germany):
George Lakoff and Mark JohnsonIn their book Philosophy in the Flesh: The Embodied Mind and its Challenge to Western Thought, philosophers George Lakoff and Mark Johnson wrote about fascism, in the chapter about morality:
John LukacsJohn Lukacs, Hungarian-American historian and Holocaust survivor, argues in The Hitler of History that there is no such thing as generic fascism, claiming that National Socialism and Italian Fascism were more different than similar and that, alongside communism, they were ultimately radical forms of populism. Ludwig von MisesClassical liberal economist and philosopher Ludwig von Mises, in his 1927 book Liberalism, argued that fascism was a nationalist and militarist reaction against the rise of the communist Third International, in which the nationalists and militarists came to oppose the principles of liberal democracy because "Liberalism, they thought, stayed their hand when they desired to strike a blow against the revolutionary parties while it was still possible to do so. If liberalism had not hindered them, they would, so they believe, have bloodily nipped the revolutionary movements in the bud. Revolutionary ideas had been able to take root and flourish only because of the tolerance they had been accorded by their opponents, whose will power had been enfeebled by a regard for liberal principles that, as events subsequently proved, was overscrupulous." He continues by defining fascism as follows:
Ernst NolteErnst Nolte, a German historian and Hegelian philosopher, defined fascism in 1965 as a reaction against other political movements, especially Marxism: "Fascism is anti-Marxism which seeks to destroy the enemy by the evolvement of a radically opposed and yet related ideology and by the use of almost identical and yet typically modified methods, always, however, within the unyielding framework of national self-assertion and autonomy." Nolte also argued that fascism functioned at three levels: in the world of politics as a form of opposition to Marxism, at the sociological level in opposition to bourgeois values, and in the "metapolitical" world as "resistance to transcendence" ("transcendence" in German can be translated as the "spirit of modernity"). Kevin PassmoreKevin Passmore, a history lecturer at Cardiff University, defines fascism in his 2002 book Fascism: A Very Short Introduction. His definition is directly descended from the view put forth by Ernesto Laclau, and is also informed by a desire to adjust for what he believes are shortcomings in Marxist, Weberian and other analyses of fascism:
Robert PaxtonRobert Paxton, a professor emeritus at Columbia University, defines fascism in his 2004 book The Anatomy of Fascism as:
In the same book, Paxton also argues that fascism's foundations lie in a set of "mobilizing passions" rather than an elaborated doctrine. He argues these passions can explain much of the behaviour of fascists:
Stanley G. PayneHistorian of fascism Stanley G. Payne created a lengthy list of characteristics to identify fascism in 1995: in summary form, there are three main strands. First, Payne's "fascist negations" refers to such typical policies as anti-communism and anti-liberalism. Second, "fascist goals" include a nationalist dictatorship and an expanded empire. Third, "fascist style", is seen in its emphasis on violence and authoritarianism, and its exultation of men above women, and young above old.
Jason StanleyIn 2020, National Public Radio interviewed Jason Stanley, a professor of philosophy at Yale University, regarding his book How Fascism Works: The Politics of Us and Them. Stanley defined fascism as "a cult of the leader who promises national restoration in the face of humiliation brought on by supposed communists, Marxists and minorities and immigrants who are supposedly posing a threat to the character and the history of a nation" and further observed that "The leader proposes that only he can solve it and all of his political opponents are enemies or traitors." Zeev SternhellZeev Sternhell, a historian and professor of political science, described Fascism as a reaction against modernity and a backlash against the changes it had caused to society, as a "rejection of the prevailing systems: liberalism and Marxism, positivism and democracy". At the same time, Sternhell argued that part of what made Fascism unique was that it wanted to retain the benefits of progress and modernism while rejecting the values and social changes that had come with it; Fascism embraced liberal market-based economics and the violent revolutionary rhetoric of Marxism, but rejected their philosophical principles. By MarxistsMarxists argue that fascism represents the last attempt of a ruling class (specifically, the capitalist bourgeoisie) to preserve its grip on power in the face of an imminent proletarian revolution. Fascist movements are not necessarily created by the ruling class, but they can only gain political power with the help of that class and with funding from big business. Once in power, the fascists serve the interests of their benefactors. György LukácsHungarian philosopher György Lukács in his works The Destruction of Reason (Die Zerstörung der Vernunft, 1952) and Zur Kritik der faschistischen Ideologie (1989) considers the ideology of fascism as the "demagogic synthesis" of all the irrationalist trends of the 19th and early 20th centuries, such as the reaction against the ideas of the Enlightenment and the French Revolution, the Romantic critique of capitalism (Carlyle) which after 1848 turned into "indirect apologetics" of capitalism (Nietzsche), anti-democratic or "aristocratic epistemology" (Lukács' term for philosophies that considered knowledge to be the privilege of an elite, first expressed in Schelling's concept of intellectual intuition and culminating in the metaphysical views of Henri Bergson), emphasis on myth and mysticism, the rejection of humanism, a cult of personality around the leader, the subjugation of reason to instinct, the conception of the nation and people in clearly biological terms, the glorification of war, etc.. According to Lukács, the historical significance of Hitler and Mussolini lies not in that they brought anything new to the ideological field, but in that they condensed all existing reactionary and irrationalist ideologies of the past and through their successful national and social demagogy brought them "from the scholar's study and intellectual coteries to the streets." Bertolt BrechtGerman playwright Bertolt Brecht describes fascism as: "a historic phase of capitalism" and "...the nakedest, most shameless, most oppressive, and most treacherous form of capitalism" (1935). Georgi DimitrovGeorgi Dimitrov, a Bulgarian Communist, was a theorist of capitalism who expanded Lenin's ideas and the work of Clara Zetkin. Delivering an official report to the 7th World Congress of the Communist Third International in August 1935, Georgi Dimitrov cited the definition of fascism formulated with the help of Clara Zetkin at the Third Plenum as "the open, terrorist dictatorship of the most reactionary, most chauvinistic, and most imperialist elements of finance capital". According to Dimitrov:
Leon TrotskyOne of Russian Marxist revolutionary Leon Trotsky’s earliest attempts at trying to define fascism was in November 1931 when he wrote a letter to a friend titled "What is Fascism". In it, Trotsky wrote, in what is as much description as analysis:
In Trotsky’s posthumously published 1944 tract, Fascism: What It Is and How to Fight It, he noted: "The historic function of fascism is to smash the working class, destroy its organizations, and stifle political liberties when the capitalists find themselves unable to govern and dominate with the help of democratic machinery." Amadeo BordigaAmadeo Bordiga argued that fascism is merely another form of bourgeois rule, on the same level as bourgeois democracy or traditional monarchy, and that it is not particularly reactionary or otherwise exceptional. Clara ZetkinAn early study of fascism was written by Clara Zetkin for the Third Enlarged Plenum of the Executive Committee of the Communist International in 1923:
By othersLaurence W. BrittIn the Spring 2003 issue of the secular humanist magazine Free Inquiry, Laurence W. Britt, who is described as "a retired international businessperson, writer, and commentator" published "Fascism Anyone?", which included a list of 14 defining characteristics of fascism. The list has since been widely circulated in both modified and unmodified forms. In a newspaper interview in 2004, Britt expanded and clarified the meaning of some of the points in his list, and discussed how they applied to the United States at that time. The headers for Britt's original list, without his sometimes extensive explanations, are:
George OrwellAnti-fascist author George Orwell describes fascism in economic terms in a 1941 essay, "Shopkeepers At War":
Writing for Tribune magazine in 1944, Orwell stated:
Franklin D. RooseveltAmerican President Franklin D. Roosevelt, who led the US into war with the fascist Axis powers, wrote about fascism:
"Fascist" as insultSome have argued that the terms fascism and fascist have become hopelessly vague since the World War II period, and that today it is little more than a pejorative used by supporters of various political views to insult their opponents. The word fascist is sometimes used to denigrate people, institutions, or groups that would not describe themselves as ideologically fascist, and that may not fall within the formal definition of the word. As a political epithet, fascist has been used in an anti-authoritarian sense to emphasize the common ideology of governmental suppression of individual freedom. In this sense, the word fascist is intended to mean oppressive, intolerant, chauvinist, genocidal, dictatorial, racist, or aggressive. George Orwell wrote in 1944:
|
A Medley of Potpourri is just what it says; various thoughts, opinions, ruminations, and contemplations on a variety of subjects.
Search This Blog
Friday, December 9, 2022
Definitions of fascism
Definition of anarchism and libertarianism
Anarchism and libertarianism, as broad political ideologies with manifold historical and contemporary meanings, have contested definitions. Their adherents have a pluralistic and overlapping tradition that makes precise definition of the political ideology difficult or impossible, compounded by a lack of common features, differing priorities of subgroups, lack of academic acceptance, and contentious, historical usage.
Overview
"Anarchism" generally refers to the anti-authoritarian (libertarian) wing of the socialist movement. "Libertarian socialism" has been a synonym for "anarchism" since 1890, as has the term "libertarian" through the mid-20th century.
The terms "anarchism" and "libertarianism" represent broad political ideologies with multiple historical and contemporary meanings. Incompatibilities within their pluralistic tradition prove difficult or impossible to reconcile into a singular set of core beliefs. The range of ideological disparities within anarchism is often paradoxical and never fully coherent. In general, anarchists are opposed to hierarchy and capitalism but differ in how they believe that change should be made.
Other complicating factors in defining "anarchism" include disagreement over its status as a political ideology and contention over the term's historical usage. Anarchism's rejection of the state and state policy largely sits outside the purview of political scientists and in some formulations, its misconstruction as the antithesis of politics contributes to its marginalization as a political ideology.
History of usage
Since the 19th century, "libertarian" has referred to advocates for freedom of the will, or anyone who generally advocated for liberty. The first person to call themselves a "libertarian" in the political sense was Joseph Déjacque in 1857. Shortly after, in 1858, he created the New York anarchist journal Le Libertaire. Anarchist Sébastien Faure used the term later in the century to differentiate between anarchists and authoritarian socialists. While the term "libertarian" has been largely synonymous with anarchism, its meaning has more recently diluted with wider adoption from ideologically disparate groups. For example, "libertarians" include both the New Left Marxists (who do not associate with authoritarian socialists or a vanguard party) and extreme liberals (primarily concerned with civil liberties). Additionally, some anarchists use "libertarian socialist" to avoid anarchism's negative connotations and emphasize its connections with socialism.
Anarchism retains a historical association with chaos and violence. In the late 1800s, prominent anarchist Peter Kropotkin noted the popular connotations of "anarchy" as a synonym for chaos and disorder, and thus a disadvantageous name for a movement. He accepted the term despite this, just as the Dutch Sea Beggars and sans-culottes had their own names conferred. Anarchists throughout the 20th century have regretted the philosophy's association with chaos, explosives, wanton violence, and marauding. These connotations endure contemporaneously through the popular media's association of black bloc property destruction with the movement. As a result of anarchy's dual definitions, the idea of a society without central authority is endemically conflated with chaos, hampering one's ability to conceive of the former positively.
The term "anarchist" is also used as an empty signifier to show abrasive disdain. The term's association with societal malady has been, in part, an intentional strategy by its detractors to discredit it. "Libertarian" saw a similar diffusion of purpose within the American libertarian movement as a wider group less studied and less interested in minimal government adopted the term, diluting the potency of its association with the strict rights-based libertarianism of Ayn Rand and Murray Rothbard. Anarcho-capitalists and those who believe in abolition of the state have occupied the fringe of the libertarian movement.
The revival of free-market ideologies during the mid-to-late 20th century came with disagreement over what to call the movement. While many of its adherents, especially in the United States, prefer "libertarian", many Conservative libertarians reject the term's association with the 1960s New Left and its connotations of libertine hedonism. The movement is divided over the use of "Conservative" as an alternative. Those who seek both economic and social liberty would be known as classical "liberals", but that term developed associations opposite of the limited government, low-taxation, minimal state advocated by the movement. Name variants of the free-market revival movement include classical liberalism, economic liberalism, free-market liberalism and neoliberalism. "Libertarian" has the most colloquial acceptance to describe a member of the movement, or "economic libertarian", based on both the ideology's primacy of economics and its distinction from libertarians of the New Left.
Though many contemporary antiglobalization activists actively identify as "anarchists", many others use anarchist principles and strategies without formally adopting the label, preferring instead terms including "antiauthoritarian", "autonomist", "libertarian socialist", or no label. These activists display anarchistic sensibilities and follow in anarchism's tradition of antiauthoritarianism, anticapitalism, antioppression, and anti-imperialism without explicitly defining themselves as ideologically anarchist.
Relation with socialism
In the 19th century, "anarchism" and "socialism" were used interchangeably, both treated as similar threats to sociopolitical order despite their differences in views towards the state. Similarly, classical anarchism is synonymous with "libertarian socialism" in their shared commitments to autonomy and freedom, decentralization, opposing hierarchy, and opposing the vanguardism of authoritarian socialism. Generally, libertarian socialism expands to include classical anarchism, council communism, Italian autonomists, and the Marxism of Luxemburg, Mattick, and Gramsci. While there are differences between each, including whether their adherents personally identify as fully "anarchist" or "Marxist", each still classifies as anti-authoritarian socialism. Classical anarchism is distinguished from general Marxism by its opposition to centralized or authoritarian organizational structures and "the dominion of man over man". Socialist-aligned forms of anarchism are also known as "social anarchism".
The terms "anarchist" and "Marxist" originally signified factions within the First International and had no theoretical basis. The Russian anti-authoritarian Mikhail Bakunin successfully argued that the International was an authoritarian organization controlled by Karl Marx, a German. The term "Marxist" first appeared in French in 1872 to associate the anti-Bakuninist group within the International. The Marxists, in return, used the pejorative "anarchist" to label the Bakuninists. These distinctions were further conflated across state lines, such that the French anarchists conflated "Marxist" with "German". A schism between "anarchist" and "socialist" affiliation was formalized with the Second International's 1896 London Congress.
The biggest divide in the definition of anarchism is between the main individualist and socialist anarchist traditions. While anarchism sits between liberalism and socialism, the definitive extent of its affiliation with either is contested. Historians (Rocker and Woodcock) have described anarchism as the confluence of liberal individualism and socialist egalitarianism. Other activists and theorists have variously argued that one tradition is "genuine anarchism" and the other tradition is oppression (Bookchin vs. anarcho-capitalists) or a combination thereof (Black). These contemporary distinctions trace to the time of early modern anarchism when Peter Kropotkin and Alexander Berkman either broke with groups or otherwise separated the traditions of communist anarchism from individualist, mutualist, and egoist anarchism. Even the very idea of the individualist–socialist divide is contested, as some types of individualist anarchism are largely socialistic. Despite these imprecise boundaries and some similarities, socialism and individualism within anarchism have a bifurcated tradition, the former associated with the history of socialism and the latter with classical liberalism and conservatism (also known as "right-libertarianism"). Even their shared belief in anti-statism does not provide a common identity, as both traditions differ in their interpretation of state-rejection in spite of the common terms.
Relation with property and capitalism
Modern American libertarians are distinguished from the dominant libertarian tradition by their relation to property and capital. While both historical libertarianism and contemporary economic libertarianism share general antipathy towards power by government authority, the latter exempts power wielded through free market capital. Historically, libertarians including Herbert Spencer and Max Stirner supported the protection of an individual's freedom from powers of government and private ownership. In contrast, modern American libertarians support freedoms on the basis of their agreement with private property rights. The abolishment of public amenities is a common theme in modern American libertarian writings.
Forms of libertarianism that put laissez-faire economics before economic equality are commonly viewed as incompatible with anarchism's tradition of egalitarianism and anti-capitalism. Anarcho-capitalism, which would abolish the state and create a fully laissez-faire economy, lies outside the social tradition of anarchism. It shares anarchism's antipathy towards the state but not anarchism's antipathy towards hierarchy, as theorists expect from anarcho-capitalist economic power relations. The ideology follows a different paradigm from anarchism and has a fundamentally different approach and goals. Despite the "anarcho" in its title, anarcho-capitalism is more closely affiliated with capitalism and right-wing libertarianism than with anarchism. Further, within laissez-faire libertarianism, some reject the designation "anarcho-capitalism", believing that "capitalism" may either refer to the laissez-faire market they support or the government-regulated system that they oppose.
Types of definition of anarchism
Anarchism scholar Paul McLaughlin studies the various definitions of anarchism in his book Anarchism and Authority. According to him, there are three common types of anarchism definition:
- etymological definitions
- anti-statist definitions
- anti-authoritarian definitions
But all fall short from providing a precise definition of anarchism.
Etymological definition
"Anarchy" derives from the Greek anarkhos, meaning "without authority" (as opposed to "without government/state"). Hence the etymological definition of anarchism as the negation of an authority. But anarchism is generally not simply a negative stance on authority but also a positive stance about how society should be structured.
Anti-statist definition
Anti-statist definitions place the focus of interest on the negation, and confrontation in the real world, of the state by anarchism. But as with the etymological definition, anarchism is much more than anti-statism, as it rejects all various forms of established authority.
The association between anti-statism and anarchism is both commonly understood and contested.
Anarchism, according to historian Peter Marshall, exists outside standards of political theory because its aims are not based on the struggle for power within the state. It is more concerned with moral and economic theory than participation in political systems and indeed often advocates against participation in such systems.
Anarchist libertarians and modern economic libertarians share opposition to the state as their only significant commonality.
Anti-authoritarian definitions
Anti-authoritarian definitions depicts the rejection of all kind of authorities. Even though these kind of definitions are much broader than the anti-statist ones, there are still handicaps. McLaughlin, who examines under a philosophical scope, claims that anti-authoritarianism is a conclusion of anarchist thought, not an a priori statement, therefore it can not be used as a definition.
Quality management system
From Wikipedia, the free encyclopedia
https://en.wikipedia.org/wiki/Quality_management_system
A quality management system (QMS) is a collection of business processes focused on consistently meeting customer requirements and enhancing their satisfaction. It is aligned with an organization's purpose and strategic direction (ISO 9001:2015). It is expressed as the organizational goals and aspirations, policies, processes, documented information, and resources needed to implement and maintain it. Early quality management systems emphasized predictable outcomes of an industrial product production line, using simple statistics and random sampling. By the 20th century, labor inputs were typically the most costly inputs in most industrialized societies, so focus shifted to team cooperation and dynamics, especially the early signaling of problems via a continual improvement cycle. In the 21st century, QMS has tended to converge with sustainability and transparency initiatives, as both investor and customer satisfaction and perceived quality are increasingly tied to these factors. Of QMS regimes, the ISO 9000 family of standards is probably the most widely implemented worldwide – the ISO 19011 audit regime applies to both and deals with quality and sustainability and their integration.
Other QMS, e.g. Natural Step, focus on sustainability issues and assume that other quality problems will be reduced as result of the systematic thinking, transparency, documentation and diagnostic discipline.
The term "Quality Management System" and the initialism "QMS" were invented in 1991 by Ken Croucher, a British management consultant working on designing and implementing a generic model of a QMS within the IT industry.
Elements
- Quality objectives
- Quality manual
- Organizational structure and responsibilities
- Data management
- Processes – including purchasing
- Product quality leading to customer satisfaction
- Continuous improvement including corrective and preventive action
- Quality instrument
- Document control
- Employee training and engagement
- Supplier quality management
Concept of quality – historical background
The concept of a quality as we think of it now first emerged from the Industrial Revolution. Previously goods had been made from start to finish by the same person or team of people, with handcrafting and tweaking the product to meet 'quality criteria'. Mass production brought huge teams of people together to work on specific stages of production where one person would not necessarily complete a product from start to finish. In the late 19th century pioneers such as Frederick Winslow Taylor and Henry Ford recognized the limitations of the methods being used in mass production at the time and the subsequent varying quality of output. Birland established Quality Departments to oversee the quality of production and rectifying of errors, and Ford emphasized standardization of design and component standards to ensure a standard product was produced. Management of quality was the responsibility of the Quality department and was implemented by Inspection of product output to 'catch' defects.
Application of statistical control came later as a result of World War production methods, which were advanced by the work done of W. Edwards Deming, a statistician, after whom the Deming Prize for quality is named. Joseph M. Juran focused more on managing for quality. The first edition of Juran's Quality Control Handbook was published in 1951. He also developed the "Juran's trilogy", an approach to cross-functional management that is composed of three managerial processes: quality planning, quality control, and quality improvement. These functions all play a vital role when evaluating quality.
Quality, as a profession and the managerial process associated with the quality function, was introduced during the second half of the 20th century and has evolved since then. Over this period, few other disciplines have seen as many changes as the quality profession.
The quality profession grew from simple control to engineering, to systems engineering. Quality control activities were predominant in the 1940s, 1950s, and 1960s. The 1970s were an era of quality engineering and the 1990s saw quality systems as an emerging field. Like medicine, accounting, and engineering, quality has achieved status as a recognized profession
As Lee and Dale (1998) state, there are many organizations that are striving to assess the methods and ways in which their overall productivity, the quality of their products and services and the required operations to achieve them are done.
Medical devices
The two primary, state of the art, guidelines for medical device manufacturer QMS and related services today are the ISO 13485 standards and the US FDA 21 CFR 820 regulations. The two have a great deal of similarity, and many manufacturers adopt QMS that is compliant with both guidelines.
ISO 13485 are harmonized with the European Union Regulation 2017/745 as well as the IVD and AIMD directives. The ISO standard is also incorporated in regulations for other jurisdictions such as Japan (JPAL) and Canada (CMDCAS).
Quality System requirements for medical devices have been internationally recognized as a way to assure product safety and efficacy and customer satisfaction since at least 1983 and were instituted as requirements in a final rule published on October 7, 1996. The U.S. Food and Drug Administration (FDA) had documented design defects in medical devices that contributed to recalls from 1983 to 1989 that would have been prevented if Quality Systems had been in place. The rule is promulgated at 21 CFR 820.
According to current Good Manufacturing Practice (GMP), medical device manufacturers have the responsibility to use good judgment when developing their quality system and apply those sections of the FDA Quality System (QS) Regulation that are applicable to their specific products and operations, in Part 820 of the QS regulation. As with GMP, operating within this flexibility, it is the responsibility of each manufacturer to establish requirements for each type or family of devices that will result in devices that are safe and effective, and to establish methods and procedures to design, produce, and distribute devices that meet the quality system requirements.
The FDA has identified in the QS regulation the 7 essential subsystems of a quality system. These subsystems include:
- Management controls;
- Design controls;
- Production and process controls
- Corrective and preventative actions
- Material controls
- Records, documents, and change controls
- Facilities and equipment controls
all overseen by management and quality audits.
Because the QS regulation covers a broad spectrum of devices and production processes, it allows some leeway in the details of quality system elements. It is left to manufacturers to determine the necessity for, or extent of, some quality elements and to develop and implement procedures tailored to their particular processes and devices. For example, if it is impossible to mix up labels at a manufacturer because there is only one label to each product, then there is no necessity for the manufacturer to comply with all of the GMP requirements under device labeling.
Drug manufacturers are regulated under a different section of the Code of Federal Regulations:
Organizations and awards
The International Organization for Standardization's ISO 9001:2015 series describes standards for a QMS addressing the principles and processes surrounding the design, development, and delivery of a general product or service. Organizations can participate in a continuing certification process to ISO 9001:2015 to demonstrate their compliance with the standard, which includes a requirement for continual (i.e. planned) improvement of the QMS, as well as more foundational QMS components such as failure mode and effects analysis (FMEA).
ISO 9000:2005 provides information on the fundamentals and vocabulary used in quality management systems. ISO 9004:2009 provides guidance on a quality management approach for the sustained success of an organization. Neither of these standards can be used for certification purposes as they provide guidance, not requirements.
The Baldrige Performance Excellence Program educates organizations in improving their performance and administers the Malcolm Baldrige National Quality Award. The Baldrige Award recognizes U.S. organizations for performance excellence based on the Baldrige Criteria for Performance Excellence. The Criteria address critical aspects of management that contribute to performance excellence: leadership; strategy; customers; measurement, analysis, and knowledge management; workforce; operations; and results.
The European Foundation for Quality Management's EFQM Excellence Model supports an award scheme similar to the Baldrige Award for European companies.
In Canada, the National Quality Institute presents the 'Canada Awards for Excellence' on an annual basis to organizations that have displayed outstanding performance in the areas of Quality and Workplace Wellness, and have met the institute's criteria with documented overall achievements and results.
The European Quality in Social Service (EQUASS) is a sector-specific quality system designed for the social services sector and addresses quality principles that are specific to service delivery to vulnerable groups, such as empowerment, rights, and person-centredness.
The Alliance for Performance Excellence is a network of state and local organizations that use the Baldrige Criteria for Performance Excellence at the grassroots level to improve the performance of local organizations and economies. browsers can find Alliance members in their state and get the latest news and events from the Baldrige community.
Process
A QMS process is an element of an organizational QMS. The ISO 9001 standard requires organizations seeking compliance or certification to define the processes which form the QMS and the sequence and interaction of these processes. Butterworth-Heinemann and other publishers have offered several books which provide step-by-step guides to those seeking the quality certifications of their products.
Examples of such processes include:
- order processes,
- production plans,
- product/ service/ process measurements to comply with specific requirements e.g. statistical process control and measurement systems analysis,
- calibrations,
- internal audit,
- corrective and preventive action,
- identification, labeling and control of non-conforming products to prevent its inadvertent use, delivery or processing,
- purchasing and related processes such as supplier selection and monitoring
ISO 9001 requires that the performance of these processes be measured, analyzed and continually improved, and the results of this form an input into the management review process.
Software
Quality management software offers the techniques, processes, structure, and resources needed to simplify manufacturing and ERP activities while handling quality concerns efficiently and cost-effectively.
Helps manufacturers to monitor, control, and document quality processes electronically to guarantee that goods are made within tolerance, meet all necessary requirements, and are defect-free. Quality management software is often used in the manufacturing industry to identify potential issues before they occur.
Some benefits of quality management software include:
- real-time data monitoring
- issue prevention
- risk management
- increased efficiency and productivity
- process consistency
- increased employee participation
Quality management software can be integrated with manufacturing execution systems (MES). A MES is a complete, dynamic software system for monitoring, tracking, documenting, and controlling the manufacturing process from raw materials to final products. When combined with QMS, these systems:
- ensure compliance
- enable quality programs
- eliminate waste
- less product recalls
- lower per-product cost
- higher product quality
- real-time information for increase in quality control
- realistic production schedules
- up-to-date inventory
- product tracking
Quality management software focuses on 4 main elements:
- Document management: Quality management software enables companies to manage all product and quality records and documents, including product specifications, work instructions, standard operating procedures (SOPs), quality policies, and training records, among other things, to fulfill highly regulated requirements. Quality management software centralizes the storage of these documents.
- Regulatory compliance: To decrease compliance risks, quality management software is used within companies to make sure they comply with ISO, OSHA, FDA, and other industry norms and requirements. The software makes closed-loop corrective and preventive action procedures (CAPA) possible, which result in faster issue resolution and issue prevention.
- Feedback loops: Quality management software permits staff to submit feedback or recommendations through centralized software. In turn, this way, managers gather insights from the shop floor creating a feedback loop.
- Training and skill management: To maintain product quality, quality management software can provide a fixed system through which employees and staff can be trained. This fixed system provides more clarity in the different tracking processes of the company and simplifies the tracking of different skill levels of employees.
Most quality management software are cloud-based and offer software as a service.
Quality management
Evolution
Quality management is a recent phenomenon but important for an organization. Civilizations that supported the arts and crafts allowed clients to choose goods meeting higher quality standards than normal goods. In societies where arts and crafts were the responsibility of master craftsmen or artists, these masters would lead studios and train and supervise others. However, the importance of craftsmen diminished as mass production and repetitive work practices were instituted. This approach’s aim was to produce large numbers of the same goods. The first proponent in the US for this approach was Eli Whitney, who proposed (interchangeable) parts manufacture for muskets, hence producing the identical components and creating a musket assembly line. The next step forward was promoted by several people including Frederick Winslow Taylor, a mechanical engineer who sought to improve industrial efficiency. He is sometimes called "the father of scientific management." He was one of the intellectual leaders of the Efficiency Movement and part of his approach laid a further foundation for quality management, including aspects like standardization and adopting improved practices. Henry Ford was also important in bringing process and quality management practices into operation in his assembly lines. In Germany, Karl Benz, often called the inventor of the motor car, was pursuing similar assembly and production practices, although real mass production was only properly initiated in Volkswagen after World War II. From this period onwards, North American companies focused predominantly upon production against lower cost with increased efficiency.
Walter A. Shewhart made a major step in the evolution towards quality management by creating a method for quality control for production, using statistical methods, first proposed in 1924. This became the foundation for his ongoing work on statistical quality control. W. Edwards Deming later applied statistical process control methods in the United States during World War II, thereby successfully improving quality in the manufacture of munitions and other strategically important products.
Quality leadership from a national perspective has changed over the past decades. After the second world war, Japan decided to make quality improvement a national imperative as part of rebuilding their economy, and sought the help of Shewhart, Deming and Juran, amongst others. W. Edwards Deming championed Shewhart's ideas in Japan from 1950 onwards. He is probably best known for his management philosophy establishing quality, productivity, and competitive position. He has formulated 14 points of attention for managers, which are a high level abstraction of many of his deep insights. They should be interpreted by learning and understanding the deeper insights. These 14 points include key concepts such as:
- Break down barriers between departments
- Management should learn their responsibilities, and take on leadership
- Supervision should be to help people and machines and gadgets to do a better job
- Improve constantly and forever the system of production and service
- Institute a vigorous program of education and self-improvement
- Drive out fear, so that everyone may work effectively for the company
In the 1950s and 1960s, Japanese goods were synonymous with cheapness and low quality, but over time their quality initiatives began to be successful, with Japan achieving high levels of quality in products from the 1970s onward. For example, Japanese cars regularly top the J.D. Power customer satisfaction ratings. In the 1980s Deming was asked by Ford Motor Company to start a quality initiative after they realized that they were falling behind Japanese manufacturers. A number of highly successful quality initiatives have been invented by the Japanese (see for example on this pages: Genichi Taguchi, QFD, Toyota Production System). Many of the methods not only provide techniques but also have associated quality culture (i.e. people factors). These methods are now adopted by the same western countries that decades earlier derided Japanese methods.
Customers recognize that quality is an important attribute in products and services. Suppliers recognize that quality can be an important differentiator between their own offerings and those of competitors (quality differentiation is also called the quality gap). In the past two decades this quality gap has been greatly reduced between competitive products and services. This is partly due to the contracting (also called outsourcing) of manufacture to countries like China and India, as well internationalization of trade and competition. These countries, among many others, have raised their own standards of quality in order to meet international standards and customer demands. The ISO 9000 series of standards are probably the best known International standards for quality management.
Some themes have become more significant including quality culture, the importance of knowledge management, and the role of leadership in promoting and achieving high quality. Disciplines like systems thinking are bringing more holistic approaches to quality so that people, process and products are considered together rather than independent factors in quality management.
Government agencies and industrial organizations that regulate products have recognized that quality culture may assist companies that produce those products. A survey of more than 60 multinational companies found that those companies whose employees rated as having a low quality culture had increased costs of $67 million/year for every 5000 employees compared to those rated as having a strong quality culture.
The influence of quality thinking has spread to non-traditional applications outside of walls of manufacturing, extending into service sectors and into areas such as sales, marketing and customer service. Statistical evidence collected in the banking sector shows a strong correlation between quality culture and competitive advantage.
Customer satisfaction has been the backbone of quality management and still is important. However, there is an expansion of the research focus from a sole customer focus towards a stakeholder focus. This is following the development of stakeholder theory. A further development of quality management is the exploration of synergies between quality management and sustainable development.
Principles
The International Standard for Quality management (ISO 9001:2015) adopts a number of management principles, that can be used by top management to guide their organizations towards improved performance.
Customer focus
The primary focus of quality management is to meet customer requirements and to strive to exceed customer expectations.
Rationale
Sustained success is achieved when an organization attracts and retains the confidence of customers and other interested parties on whom it depends. Every aspect of customer interaction provides an opportunity to create more value for the customer. Understanding current and future needs of customers and other interested parties contributes to sustained success of an organization
Leadership
Leaders at all levels establish unity of purpose and direction and create conditions in which people are engaged in achieving the organization’s quality objectives. Leadership has to take up the necessary changes required for quality improvement and encourage a sense of quality throughout organisation.
Rationale
Creation of unity of purpose and direction and engagement of people enable an organization to align its strategies, policies, processes and resources to achieve its objectives
Engagement of people
Competent, empowered and engaged people at all levels throughout the organization are essential to enhance its capability to create and deliver value.
Rationale
To manage an organization effectively and efficiently, it is important to involve all people at all levels and to respect them as individuals. Recognition, empowerment and enhancement of competence facilitate the engagement of people in achieving the organization’s quality objectives.
Process approach
Consistent and predictable results are achieved more effectively and efficiently when activities are understood and managed as interrelated processes that function as a coherent system.
Rationale
The quality management system consists of interrelated processes. Understanding how results are produced by this system enables an organization to optimize the system and its performance.
Improvement
Successful organizations have an ongoing focus on improvement.
Rationale
Improvement is essential for an organization to maintain current levels of performance, to react to changes in its internal and external conditions and to create new opportunities.
Evidence based decision making
Decisions based on the analysis and evaluation of data and information are more likely to produce desired results.
Rationale
Decision making can be a complex process, and it always involves some uncertainty. It often involves multiple types and sources of inputs, as well as their interpretation, which can be subjective. It is important to understand cause-and-effect relationships and potential unintended consequences. Facts, evidence and data analysis lead to greater objectivity and confidence in decision making.
Relationship management
For sustained success, an organization manages its relationships with interested parties, such as suppliers, retailers.
Rationale
Interested parties influence the performance of an organizations and industry. Sustained success is more likely to be achieved when the organization manages relationships with all of its interested parties to optimize their impact on its performance. Relationship management with its supplier and partner networks is of particular importance.
Criticism
The social scientist Bettina Warzecha (2017) describes the central concepts of Quality Management (QM), such as e.g. process orientation, controllability, and zero defects as modern myths. She alleges that zero-error processes and the associated illusion of controllability involve the epistemological problem of self-referentiality. The emphasis on the processes in QM also ignores the artificiality and thus arbitrariness of the difference between structure and process. Above all, the complexity of management cannot be reduced to standardized (mathematical) procedures. According to her, the risks and negative side effects of QM are usually greater than the benefits (see also brand eins, 2010).
Quality improvement and more
There are many methods for quality improvement. These cover product improvement, process improvement and people based improvement. In the following list are methods of quality management and techniques that incorporate and drive quality improvement:
- ISO 9004 — guidelines for performance improvement.
- ISO 9001 - a certified quality management system (QMS) for organisations who want to prove their ability to consistently provide products and services that meet the needs of their customers and other relevant stakeholders.
- ISO 15504-4: 2005 — information technology — process assessment — Part 4: Guidance on use for process improvement and process capability determination.
- QFD — quality function deployment, also known as the house of quality approach.
- Kaizen — 改善, Japanese for change for the better; the common English term is continuous improvement.
- Zero Defect Program — created by NEC Corporation of Japan, based upon statistical process control and one of the inputs for the inventors of Six Sigma.
- Six Sigma — 6σ, Six Sigma combines established methods such as statistical process control, design of experiments and failure mode and effects analysis (FMEA) in an overall framework.
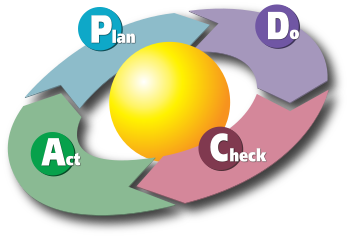
- PDCA — plan, do, check, act cycle for quality control purposes. (Six Sigma's DMAIC method (define, measure, analyze, improve, control) may be viewed as a particular implementation of this.)
- Quality circle — a group (people oriented) approach to improvement.
- Taguchi methods — statistical oriented methods including quality robustness, quality loss function, and target specifications.
- The Toyota Production System — reworked in the west into lean manufacturing.
- Kansei Engineering — an approach that focuses on capturing customer emotional feedback about products to drive improvement.
- TQM — total quality management is a management strategy aimed at embedding awareness of quality in all organizational processes. First promoted in Japan with the Deming prize which was adopted and adapted in USA as the Malcolm Baldrige National Quality Award and in Europe as the European Foundation for Quality Management award (each with their own variations).
- TRIZ — meaning "theory of inventive problem solving"
- BPR — business process reengineering, a management approach aiming at optimizing the workflows and processes within an organisation.
- OQRM — Object-oriented Quality and Risk Management, a model for quality and risk management.
- Top Down & Bottom Up Approaches—Leadership approaches to change
Proponents of each approach have sought to improve them as well as apply them for small, medium and large gains. Simple one is Process Approach, which forms the basis of ISO 9001:2008 Quality Management System standard, duly driven from the 'Eight principles of Quality management', process approach being one of them. Thareja writes about the mechanism and benefits: "The process (proficiency) may be limited in words, but not in its applicability. While it fulfills the criteria of all-round gains: in terms of the competencies augmented by the participants; the organisation seeks newer directions to the business success, the individual brand image of both the people and the organisation, in turn, goes up. The competencies which were hitherto rated as being smaller, are better recognized and now acclaimed to be more potent and fruitful". The more complex Quality improvement tools are tailored for enterprise types not originally targeted. For example, Six Sigma was designed for manufacturing but has spread to service enterprises. Each of these approaches and methods has met with success but also with failures.
Some of the common differentiators between success and failure include commitment, knowledge and expertise to guide improvement, scope of change/improvement desired (Big Bang type changes tend to fail more often compared to smaller changes) and adaption to enterprise cultures. For example, quality circles do not work well in every enterprise (and are even discouraged by some managers), and relatively few TQM-participating enterprises have won the national quality awards.
There have been well publicized failures of BPR, as well as Six Sigma. Enterprises therefore need to consider carefully which quality improvement methods to adopt, and certainly should not adopt all those listed here.
It is important not to underestimate the people factors, such as culture, in selecting a quality improvement approach. Any improvement (change) takes time to implement, gain acceptance and stabilize as accepted practice. Improvement must allow pauses between implementing new changes so that the change is stabilized and assessed as a real improvement, before the next improvement is made (hence continual improvement, not continuous improvement).
Improvements that change the culture take longer as they have to overcome greater resistance to change. It is easier and often more effective to work within the existing cultural boundaries and make small improvements (that is 'Kaizen') than to make major transformational changes. Use of Kaizen in Japan was a major reason for the creation of Japanese industrial and economic strength.
On the other hand, transformational change works best when an enterprise faces a crisis and needs to make major changes in order to survive. In Japan, the land of Kaizen, Carlos Ghosn led a transformational change at Nissan Motor Company which was in a financial and operational crisis. Well organized quality improvement programs take all these factors into account when selecting the quality improvement methods.
Quality standards
ISO standards
The International Organization for Standardization (ISO) is an independent non-governmental coalition representing 165 countries through their national standards bodies. ISO brings together experts to share knowledge and develop voluntary, consensus-based international commercial, industrial and technical standards.
ISO created Quality Management System (QMS) standards in 1987. They were the ISO 9000:1987 series of standards comprising ISO 9001:1987, ISO 9002:1987 and ISO 9003:1987; which were applicable in different types of industries, based on the type of activity or process: designing, production or service delivery.
The standards are reviewed every few years by the International Organization for Standardization. The version in 1994 was called the ISO 9000:1994 series; consisting of the ISO 9001:1994, 9002:1994 and 9003:1994 versions.
A major revision was published in the year 2000 and the series was called ISO 9000:2000 series. The ISO 9002 and 9003 standards were integrated into one single certifiable standard: ISO 9001:2000. After December 2003, organizations holding ISO 9002 or 9003 standards had to complete a transition to the new standard.
ISO released a minor revision, ISO 9001:2008 on 14 October 2008. It contains no new requirements. Many of the changes were to improve consistency in grammar, facilitating translation of the standard into other languages for use by over 950,000 certified organization in the 175 countries (as at Dec 2007) that use the standard.
The ISO 9004:2009 document gives guidelines for performance improvement over and above the basic standard (ISO 9001:2000). This standard provides a measurement framework for improved quality management, similar to and based upon the measurement framework for process assessment.
The last major revision was published 15 September 2015. This change adopted the High Level Structure, contained in ISO Directive 1 Annex SL, for the first time.
The Quality Management System standards created by ISO are meant for certification of the processes and management arrangements of an organization, not the product or service itself. The ISO 9000 family of standards do not set out requirements for product or service approval. Instead, ISO 9001 requires that product or service requirements are agreed between the organization and its customers, and that the organization manages its business processes to achieve these agreed requirements.
ISO 9001 states that the Quality Management System requirements of the standard are generic and are intended to be applicable to any organization, regardless of its type or size, or the products and services it provides, however, ISO has also published a number of separate standards which specify Quality Management System requirements for specific industries, in many cases those involved in the production or processing of goods typically regulated by nations and other global jurisdictions, in order to ensure that unique elements pertaining to public health and safety are integrated into these Quality Management Systems.
ISO 13485 specifies Quality Management System requirements for organizations involved in the design and manufacture of medical devices in order to demonstrate the ability to meet relevant regulatory requirements. Such organizations can be involved in one or more stages of the life-cycle, including design and development, production, storage and distribution, installation, or servicing of a medical device and design and development or provision of associated activities (e.g. technical support). ISO 13485 can also be used by suppliers or external parties that provide product, including quality management system-related services to such organizations. ISO has not published a standard in similar manner specifying Quality Management System requirements unique to the pharmaceutical industry for regulatory purposes, therefore compliance with ISO 9001 is typically utilized by organizations involved in the design and manufacture of pharmaceuticals.
In 2005 ISO published ISO 22000 specifying the Food Safety Management System requirements for the food industry. This standard covers the values and principles of ISO 9000 and the HACCP standards. It gives one single integrated standard for the food industry, defining requirements for any organization in the food chain.
Technical Standard TS 16949 defines requirements in addition to those in ISO 9001:2008 specifically for the automotive industry.
ISO has a number of standards that support quality management. One group describes processes (including ISO/IEC 12207 and ISO/IEC 15288) and another describes process assessment and improvement ISO 15504.
CMMI and IDEAL methods
The Software Engineering Institute has its own process assessment and improvement methods, called CMMI (Capability Maturity Model Integration) and IDEAL respectively.
Capability Maturity Model Integration (CMMI) is a process improvement training and appraisal program and service administered and marketed by Carnegie Mellon University and required by many DOD and U.S. Government contracts, especially in software development. Carnegie Mellon University claims CMMI can be used to guide process improvement across a project, division, or an entire organization. Under the CMMI methodology, processes are rated according to their maturity levels, which are defined as: Initial, Managed, Defined, Quantitatively Managed, Optimizing. Currently supported is CMMI Version 1.3. CMMI is registered in the U.S. Patent and Trademark Office by Carnegie Mellon University.
Three constellations of CMMI are:
- Product and service development (CMMI for Development)
- Service establishment, management, and delivery (CMMI for Services)
- Product and service acquisition (CMMI for Acquisition).
CMMI Version 1.3 was released on November 1, 2010. This release is noteworthy because it updates all three CMMI models (CMMI for Development, CMMI for Services, and CMMI for Acquisition) to make them consistent and to improve their high maturity practices. The CMMI Product Team has reviewed more than 1,150 change requests for the models and 850 for the appraisal method.
As part of its mission to transition mature technology to the software community, the SEI has transferred CMMI-related products and activities to the CMMI Institute, a 100%-controlled subsidiary of Carnegie Innovations, Carnegie Mellon University’s technology commercialization enterprise.
Quality management software
Quality Management Software is a category of technologies used by organizations to manage the delivery of high quality products. Solutions range in functionality, however, with the use of automation capabilities they typically have components for managing internal and external risk, compliance, and the quality of processes and products. Pre-configured and industry-specific solutions are available and generally require integration with existing IT architecture applications such as ERP, SCM, CRM, and PLM.
Quality Management Software Functionalities
- Non-Conformances/Corrective and Preventive Action
- Compliance/Audit Management
- Risk Management
- Statistical Process Control
- Failure Mode and Effects Analysis
- Complaint Handling
- Advanced Product Quality Planning
- Environment, Health, and Safety
- Hazard Analysis & Critical Control Points
- Production Part Approval Process
Enterprise Quality Management Software
The intersection of technology and quality management software prompted the emergence of a new software category: Enterprise Quality Management Software (EQMS). EQMS is a platform for cross-functional communication and collaboration that centralizes, standardizes, and streamlines quality management data from across the value chain. The software breaks down functional silos created by traditionally implemented standalone and targeted solutions. Supporting the proliferation and accessibility of information across supply chain activities, design, production, distribution, and service, it provides a holistic viewpoint for managing the quality of products and processes.
Quality terms
- Quality Improvement can be distinguished from Quality Control in that Quality Improvement is the purposeful change of a process to improve the reliability of achieving an outcome.
- Quality Control is the ongoing effort to maintain the integrity of a process to maintain the reliability of achieving an outcome.
- Quality Assurance is the planned or systematic actions necessary to provide enough confidence that a product or service will satisfy the given requirements.
Computational neuroscience
From Wikipedia, the free encyclopedia https://en.wikipedia.org/wiki/Computational_neuroscience Computatio...
-
From Wikipedia, the free encyclopedia Ward Cunningham , inventor of the wiki A wiki is a website on whi...
-
From Wikipedia, the free encyclopedia https://en.wikipedia.org/wiki/Human_cloning Diagram of ...
-
From Wikipedia, the free encyclopedia Islamic State of Iraq and the Levant الدولة الإسلامية في العراق والشام ( ...