Antimicrobial preservatives
Antimicrobial
preservatives prevent degradation by bacteria. This method is the most
traditional and ancient type of preserving—ancient methods such as
pickling and adding honey prevent microorganism growth by modifying the
pH level. The most commonly used antimicrobial preservative is lactic acid. Common antimicrobial preservatives are presented in the table. Nitrates and nitrites are also antimicrobial.
The detailed mechanism of these chemical compounds range from
inhibiting growth of the bacteria to the inhibition of specific enzymes.
Water-based home and personal care products use broad-spectrum preservatives, such as isothiazolinones and formaldehyde releasers, which may cause sensitization, allergic skin reactions, and toxicity to aquatic life.
| E number | chemical compound | comment |
|---|---|---|
| E200 – E203 | sorbic acid, sodium sorbate and sorbates | common for cheese, wine, baked goods, personal care products |
| E210 – E213 | benzoic acid, sodium benzoate and benzoates | used in acidic foods such as jams, salad dressing, juices, pickles, carbonated drinks, soy sauce |
| E214 – E219 | hydroxybenzoate and derivatives, "parabens" | stable at a broad pH range, personal care products |
| E220 – E227 | sulfur dioxide and sulfites | common for fruits, wine |
| E249 – E250 | nitrite | used in meats to prevent botulism toxin |
| E251 – E252 | nitrate | used in meats |
| E270 | lactic acid | - |
| E280 – E283 | propionic acid and sodium propionate | baked goods |
| n/a | isothiazolinones (MIT, CMIT, BIT) | home and personal care products, paints/coatings |
| n/a | formaldehyde releasers (DMDM hydantoin) | home and personal care products |
Antioxidants
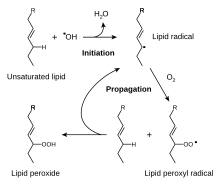
The free radical pathway for the first phase of the oxidative rancidification of fats. This process is slowed by antioxidants.
The oxidation process spoils most food, especially those with a high fat content. Fats quickly turn rancid when exposed to oxygen. Antioxidants prevent or inhibit the oxidation process. The most common antioxidant additives are ascorbic acid (vitamin C) and ascorbates. Thus, antioxidants are commonly added to oils, cheese, and chips. Other antioxidants include the phenol derivatives BHA, BHT, TBHQ and propyl gallate. These agents suppress the formation of hydroperoxides. Other preservatives include ethanol and methylchloroisothiazolinone.
| E number | chemical compound | comment |
|---|---|---|
| E300-304 | ascorbic acid, sodium ascorbate | cheese, chips |
| E321 | butylated hydroxytoluene, butylated hydroxyanisole | also used in food packaging |
| E310-312 | gallic acid and sodium gallate | oxygen scavenger |
| E220 – E227 | sulfur dioxide and sulfites | beverages, wine |
| E306 – E309 | tocopherols | vitamin E activity |
A variety of agents are added to sequester (deactivate) metal ions
that otherwise catalyze the oxidation of fats. Common sequestering
agents are disodium EDTA, citric acid (and citrates), tartaric acid, and lecithin.
Nonsynthetic compounds for food preservation
Citric and ascorbic acids target enzymes that degrade fruits and vegetables, e.g., mono/polyphenol oxidase which turns surfaces of cut apples and potatoes brown. Ascorbic acid and tocopherol, which are vitamins, are common preservatives. Smoking entails exposing food to a variety of phenols, which are antioxidants. Natural preservatives include rosemary and oregano extract, hops, salt, sugar, vinegar, alcohol, diatomaceous earth and castor oil.
Traditional preservatives, such as sodium benzoate have raised
health concerns in the past. Benzoate was shown in a study to cause
hypersensitivity in some asthma sufferers. This has caused reexamination
of natural preservatives which occur in vegetables.
History and methods
Preservatives have been used since prehistoric times. Smoked meat for example has phenols
and other chemicals that delay spoilage. The preservation of foods has
evolved greatly over the centuries and has been instrumental in
increasing food security. The use of preservatives other than
traditional oils, salts, paints, etc. in food began in the late 19th
century, but was not widespread until the 20th century.
The use of food preservatives varies greatly depending on the
country. Many developing countries that do not have strong governments
to regulate food additives face either harmful levels of preservatives
in foods or a complete avoidance of foods that are considered unnatural
or foreign. These countries have also proven useful in case studies
surrounding chemical preservatives, as they have been only recently
introduced.
In urban slums of highly populated countries, the knowledge about
contents of food tends to be extremely low, despite consumption of these
imported foods.
Drying
In ancient times the sun and wind naturally dried out foods. Middle
Eastern and Oriental cultures started drying foods in 1,200 B.C. in the
sun. The Romans used a lot of dry fruit. In the Middle Ages, people made
“still houses” where fruits, vegetables, and herbs could dry out in
climates that did not have strong sunlight. Sometimes fires were made to
create heat to dry foods. Drying prevents yeasts and bread molds (Rhizopus) from growing by removing moisture so bacteria cannot grow.
Freezing
Cellars, caves, and cool streams were used for freezing. American
estates had ice houses built to store ice and food on the ice. The
icehouse was then converted to an “icebox”. The Icebox was converted in the 1800s to mechanical refrigeration. Clarence Birdseye found in the 1800s that freezing meats and vegetables at a low temperature made them taste better.
Fermenting
Fermenting was discovered when a few grains of barley were left in the rain and turned into beer. Microorganisms ferment the starch-derived sugars into alcohols. This is also how fruits are fermented into wine and cabbage into Kimchi
or sauerkraut. Anthropologists believe that as early as 10,000 B.C
people began to settle and grow barley. They began to make beer and
believed that it was a gift from gods. It was used to preserve foods and
to create more nutritious foods from less desirable ingredients.
Vitamins are produced through fermentation by microorganisms making the end product more nutritious.
Pickling
Pickling occurs when foods are placed in a container with vinegar or
another acid. It is thought that pickling came about when people used to
place food in wine or beer to preserve it due to them having a low pH. Containers had to be stoneware or glass (vinegar will dissolve metal from pots). After the food was eaten, the pickling brine had other uses. Romans would make a concentrated pickle sauce called “garum”.
It was very concentrated and the dish that it would be used in would
only need a few drops to get the fish taste. Due to new foods arriving
from Europe in the 16th century, food preservation increased. Ketchup
originated from Europe as an oriental fish brine and when it made it to
America, sugar was added. Pickling sauces were soon part of many recipes
such as chutneys, relish, piccalilli, mustard, and ketchup when
different spices were added to them.
Curing
The beginning of curing was done through dehydration. Salting was
used by early cultures to help desiccate foods. Many different salts
were used from different places such as rock salt, sea salt, spiced
salt, etc.. People began to experiment and found in the 1800s that some
salts gave meat an appealing red color instead of the grey that they
were used to. During their experimenting in the 1920s they realized this
mixture of salts were nitrates (saltpeter) that prevented Clostridium botulinum growth.
Jam and Jelly
Early cultures also used honey or sugar as a preservatives. Greece used a quince
and honey mixture with a slight amount of drying and then tightly
packed into jars. The Romans used the same technique but instead cooked
the honey and quince mixture to make a solid texture. Indian and Oriental traders brought sugarcane to the northern climates where housewives were then able to make preservatives by heating fruit with the sugarcane.
Canning
Canning started in 1790 from a French confectioner, Nicolas Appert,
when he found that by applying heat to food in sealed glass bottles,
the food is free from spoilage. Appert’s ideas were tried by the French
Navy with meat, vegetables, fruit, and milk in 1806. An Englishman, Peter Durand
decided to use Appert’s method on tin cans in 1810. Even though Appert
found a method that worked, he did not understand why it worked because
many believed that the lack of air caused the preservation. In 1864 Louis Pasteur linked food spoilage/illness to microorganisms. Different foods are placed into jars or cans and heated to a microorganism and enzyme inactivating temperature. They are then cooled forming a vacuum seal which prevents microorganisms from contaminating the foods.
Public awareness of food preservation
Public awareness of food preservatives is uneven.
Americans have a perception that food-borne illnesses happen more often
in other countries. This may be true, but the occurrence of illnesses,
hospitalizations, and deaths are still high. It is estimated by the Center for Disease Control (CDC) that each year there are 76 million illnesses, 325,000 hospitalizations, and 5,000 deaths linked to food-borne illness.
The increasing demand for ready-to-eat fresh food products has
led to challenges for food distributors regarding the safety and quality
of their foods. Artificial preservatives meet some of these challenges
by preserving freshness for longer periods of time, but these
preservatives can cause negative side-effects as well. Sodium nitrite is a preservative used in lunch meats, hams, sausages, hot dogs, and bacon to prevent botulism. It serves the important function of controlling the bacteria that cause botulism, but sodium nitrite can react with proteins, or during cooking at high heats, to form carcinogenic N-nitrosamines. It has also been linked to cancer in lab animals. The commonly used sodium benzoate has been found to extend the shelf life of bottled tomato paste to 40 weeks without loss of quality. However, it can form the carcinogen benzene when combined with vitamin C. Many food manufacturers have reformed their products to eliminate this combination, but a risk still exists. Consumption of sodium benzoate may also cause hyperactivity. For over 30 years, there has been a debate about whether or not preservatives and other food additives can cause hyperactivity. Studies have found that there may be increases in hyperactivity amongst children who consume artificial colorings and benzoate preservatives and who are already genetically predisposed to hyperactivity, but these studies were not entirely conclusive. Hyperactivity
only increased moderately, and it was not determined if the
preservatives, colorings, or a combination of the two were responsible
for the increase.