From Wikipedia, the free encyclopedia
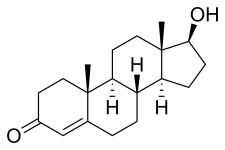
Testosterone
|

|
| Names
|
| IUPAC name
17β-Hydroxyandrost-4-en-3-one
|
Preferred IUPAC name
(1S,3aS,3bR,9aR,9bS,11aS)-1-Hydroxy-9a,11a-dimethyl-1,2,3,3a,3b,4,5,8,9,9a,9b,10,11,11a-tetradecahydro-7H-cyclopenta[a]phenanthren-7-one |
| Other names
Androst-4-en-17β-ol-3-one
|
| Identifiers
|
|
|
|
|
|
|
| ChEBI
|
|
| ChEMBL
|
|
| ChemSpider
|
|
| DrugBank
|
|
| ECHA InfoCard
|
100.000.336 
|
| KEGG
|
|
|
|
|
| UNII
|
|
|
|
|
|
|
|
| Properties
|
|
|
C19H28O2
|
| Molar mass
|
288.431 g·mol−1
|
| Melting point
|
151.0 °C (303.8 °F; 424.1 K)
|
| Pharmacology
|
|
|
G03BA03 (WHO)
|
| License data
|
|
|
|
Transdermal (gel, cream, solution, patch), by mouth (as testosterone undecanoate), in the cheek, intranasal (gel), intramuscular injection (as esters), subcutaneous pellets
|
| Pharmacokinetics:
|
|
|
Oral: very low (due to extensive first pass metabolism)
|
|
|
97.0–99.5% (to SHBG and albumin)
|
|
|
Liver (mainly reduction and conjugation)
|
|
|
2–4 hours
|
|
|
Urine (90%), feces (6%)
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
Testosterone is the primary sex hormone and anabolic steroid in males. In male humans, testosterone plays a key role in the development of male reproductive tissues such as testes and prostate, as well as promoting secondary sexual characteristics such as increased muscle and bone mass, and the growth of body hair. In addition, testosterone in both sexes is involved in health and well-being and in the prevention of osteoporosis. Insufficient levels of testosterone in men may lead to abnormalities including frailty and bone loss.
Testosterone is a steroid from the androstane class containing a keto and hydroxyl groups at positions three and seventeen respectively. It is biosynthesized in several steps from cholesterol and is converted in the liver to inactive metabolites. It exerts its action through binding to and activation of the androgen receptor. In humans and most other vertebrates, testosterone is secreted primarily by the testicles of males and, to a lesser extent, the ovaries of females. On average, in adult males, levels of testosterone are about seven to eight times as great as in adult females. As the metabolism of testosterone in males is more pronounced, the daily production is about 20 times greater in men. Females are also more sensitive to the hormone.
In addition to its role as a natural hormone, testosterone is used as a medication in the treatment of hypogonadism in men and breast cancer in women. Since testosterone levels decrease as men age, testosterone is sometimes used in older men to counteract this deficiency. It is also used as part of transgender hormone therapy for transgender men and illicitly to enhance physique and performance, for instance in athletes.
Biological effects
In general, androgens such as testosterone promote protein synthesis and thus growth of tissues with androgen receptors. Testosterone can be described as having virilising and anabolic
effects (though these categorical descriptions are somewhat arbitrary,
as there is a great deal of mutual overlap between them).
Testosterone effects can also be classified by the age of usual occurrence. For postnatal effects in both males and females, these are mostly dependent on the levels and duration of circulating free testosterone.
Before birth
Effects before birth are divided into two categories, classified in relation to the stages of development.
The first period occurs between 4 and 6 weeks of the gestation. Examples include genital virilisation such as midline fusion, phallic urethra, scrotal thinning and rugation, and phallic enlargement; although the role of testosterone is far smaller than that of dihydrotestosterone. There is also development of the prostate gland and seminal vesicles.
During the second trimester, androgen level is associated with sex formation.
Specifically, testosterone, along with anti-Müllerian hormone (AMH)
promote growth of the Wolffian duct and degeneration of the Müllerian
duct respectively.
This period affects the femininization or masculinization of the fetus
and can be a better predictor of feminine or masculine behaviours such
as sex typed behaviour than an adult's own levels. Prenatal androgens
apparently influence interests and engagement in gendered activities and
have moderate effects on spatial abilities. Among women with CAH,
a male-typical play in childhood correlated with reduced satisfaction
with the female gender and reduced heterosexual interest in adulthood.
Early infancy
Early
infancy androgen effects are the least understood. In the first weeks
of life for male infants, testosterone levels rise. The levels remain in
a pubertal range for a few months, but usually reach the barely
detectable levels of childhood by 4–7 months of age. The function of this rise in humans is unknown. It has been theorized that brain masculinization is occurring since no significant changes have been identified in other parts of the body. The male brain is masculinized by the aromatization of testosterone into estrogen, which crosses the blood–brain barrier and enters the male brain, whereas female fetuses have α-fetoprotein, which binds the estrogen so that female brains are not affected.
Before puberty
Before puberty effects of rising androgen levels occur in both boys and girls. These include adult-type body odor, increased oiliness of skin and hair, acne, pubarche (appearance of pubic hair), axillary hair (armpit hair), growth spurt, accelerated bone maturation, and facial hair.
Pubertal
Pubertal
effects begin to occur when androgen has been higher than normal adult
female levels for months or years. In males, these are usual late
pubertal effects, and occur in women after prolonged periods of
heightened levels of free testosterone in the blood. The effects
include:
Growth of spermatogenic tissue in testicles, male fertility, penis or clitoris enlargement, increased libido and frequency of erection or clitoral engorgement occurs. Growth of jaw, brow, chin, and nose and remodeling of facial bone contours, in conjunction with human growth hormone occurs. Completion of bone maturation and termination of growth. This occurs indirectly via estradiol metabolites
and hence more gradually in men than women. Increased muscle strength
and mass, shoulders become broader and rib cage expands, deepening of
voice, growth of the Adam's apple. Enlargement of sebaceous glands. This might cause acne, subcutaneous fat in face decreases. Pubic hair extends to thighs and up toward umbilicus, development of facial hair (sideburns, beard, moustache), loss of scalp hair (androgenetic alopecia), increase in chest hair, periareolar hair, perianal hair, leg hair, armpit hair.
Adult
Testosterone is necessary for normal sperm development. It activates genes in Sertoli cells, which promote differentiation of spermatogonia. It regulates acute HPA (hypothalamic–pituitary–adrenal axis) response under dominance challenge. Androgen including testosterone enhances muscle growth. Testosterone also regulates the population of thromboxane A2 receptors on megakaryocytes and platelets and hence platelet aggregation in humans.
Adult testosterone effects are more clearly demonstrable in males
than in females, but are likely important to both sexes. Some of these
effects may decline as testosterone levels might decrease in the later
decades of adult life.
Health risks
Testosterone does not appear to increase the risk of developing prostate cancer.
In people who have undergone testosterone deprivation therapy,
testosterone increases beyond the castrate level have been shown to
increase the rate of spread of an existing prostate cancer.
Conflicting results have been obtained concerning the importance of testosterone in maintaining cardiovascular health.
Nevertheless, maintaining normal testosterone levels in elderly men has
been shown to improve many parameters that are thought to reduce
cardiovascular disease risk, such as increased lean body mass, decreased
visceral fat mass, decreased total cholesterol, and glycemic control.
High androgen levels are associated with menstrual cycle irregularities in both clinical populations and healthy women.
Sexual arousal
Testosterone levels follow a nycthemeral rhythm that peaks early each day, regardless of sexual activity.
There are positive correlations between positive orgasm
experience in women and testosterone levels where relaxation was a key
perception of the experience. There is no correlation between
testosterone and men's perceptions of their orgasm experience, and also
no correlation between higher testosterone levels and greater sexual
assertiveness in either sex.
Sexual arousal and masturbation in women produce small increases in testosterone concentrations. The plasma levels of various steroids significantly increase after masturbation in men and the testosterone levels correlate to those levels.
Mammalian studies
Studies
conducted in rats have indicated that their degree of sexual arousal is
sensitive to reductions in testosterone. When testosterone-deprived
rats were given medium levels of testosterone, their sexual behaviours
(copulation, partner preference, etc.) resumed, but not when given low
amounts of the same hormone. Therefore, these mammals may provide a
model for studying clinical populations among humans suffering from
sexual arousal deficits such as hypoactive sexual desire disorder.
Every mammalian species examined demonstrated a marked increase in a male's testosterone level upon encountering a novel female. The reflexive testosterone increases in male mice is related to the male's initial level of sexual arousal.
In non-human primates, it may be that testosterone in puberty
stimulates sexual arousal, which allows the primate to increasingly seek
out sexual experiences with females and thus creates a sexual
preference for females.
Some research has also indicated that if testosterone is eliminated in
an adult male human or other adult male primate's system, its sexual
motivation decreases, but there is no corresponding decrease in ability
to engage in sexual activity (mounting, ejaculating, etc.).
In accordance with sperm competition
theory, testosterone levels are shown to increase as a response to
previously neutral stimuli when conditioned to become sexual in male
rats.
This reaction engages penile reflexes (such as erection and
ejaculation) that aid in sperm competition when more than one male is
present in mating encounters, allowing for more production of successful
sperm and a higher chance of reproduction.
Males
In men, higher levels of testosterone are associated with periods of sexual activity.
Men who watch a sexually explicit movie have an average increase
of 35% in testosterone, peaking at 60–90 minutes after the end of the
film, but no increase is seen in men who watch sexually neutral films. Men who watch sexually explicit films also report increased motivation, competitiveness, and decreased exhaustion. A link has also been found between relaxation following sexual arousal and testosterone levels.
Men's levels of testosterone, a hormone known to affect men's
mating behaviour, changes depending on whether they are exposed to an ovulating
or nonovulating woman's body odour. Men who are exposed to scents of
ovulating women maintained a stable testosterone level that was higher
than the testosterone level of men exposed to nonovulation cues. Men are
heavily aware of hormone cycles in females. This may be linked to the ovulatory shift hypothesis,
where males are adapted to respond to the ovulation cycles of females
by sensing when they are most fertile and whereby females look for
preferred male mates when they are the most fertile; both actions may be
driven by hormones.
Females
Androgens may modulate the physiology of vaginal tissue and contribute to female genital sexual arousal.
Women's level of testosterone is higher when measured pre-intercourse
vs pre-cuddling, as well as post-intercourse vs post-cuddling.
There is a time lag effect when testosterone is administered, on
genital arousal in women. In addition, a continuous increase in vaginal
sexual arousal may result in higher genital sensations and sexual
appetitive behaviors.
When females have a higher baseline level of testosterone, they
have higher increases in sexual arousal levels but smaller increases in
testosterone, indicating a ceiling effect on testosterone levels in
females. Sexual thoughts also change the level of testosterone but not
the level of cortisol in the female body, and hormonal contraceptives
may affect the variation in testosterone response to sexual thoughts.
Testosterone may prove to be an effective treatment in female sexual arousal disorders, and is available as a dermal patch. There is no FDA approved androgen preparation for the treatment of androgen insufficiency; however, it has been used as an off-label use to treat low libido and sexual dysfunction in older women. Testosterone may be a treatment for postmenopausal women as long as they are effectively estrogenized.
Romantic relationships
Falling in love
decreases men's testosterone levels while increasing women's
testosterone levels. There has been speculation that these changes in
testosterone result in the temporary reduction of differences in
behavior between the sexes.
However, it is suggested that after the "honeymoon phase" ends—about
four years into a relationship—this change in testosterone levels is no
longer apparent. Men who produce less testosterone are more likely to be in a relationship or married, and men who produce more testosterone are more likely to divorce. Marriage or commitment could cause a decrease in testosterone levels.
Single men who have not had relationship experience have lower
testosterone levels than single men with experience. It is suggested
that these single men with prior experience are in a more competitive
state than their non-experienced counterparts.
Married men who engage in bond-maintenance activities such as spending
the day with their spouse and/or child have no different testosterone
levels compared to times when they do not engage in such activities.
Collectively, these results suggest that the presence of competitive
activities rather than bond-maintenance activities are more relevant to
changes in testosterone levels.
Men who produce more testosterone are more likely to engage in extramarital sex.
Testosterone levels do not rely on physical presence of a partner;
testosterone levels of men engaging in same-city and long-distance
relationships are similar.
Physical presence may be required for women who are in relationships
for the testosterone–partner interaction, where same-city partnered
women have lower testosterone levels than long-distance partnered women.
Fatherhood
Fatherhood
decreases testosterone levels in men, suggesting that the emotions and
behaviour tied to decreased testosterone promote paternal care. In
humans and other species that utilize allomaternal care, paternal investment in offspring is beneficial to said offspring's survival because it allows the parental dyad
to raise multiple children simultaneously. This increases the
reproductive fitness of the parents because their offspring are more
likely to survive and reproduce. Paternal care increases offspring
survival due to increased access to higher quality food and reduced
physical and immunological threats.
This is particularly beneficial for humans since offspring are
dependent on parents for extended periods of time and mothers have
relatively short inter-birth intervals.
While the extent of paternal care varies between cultures, higher
investment in direct child care has been seen to be correlated with
lower average testosterone levels as well as temporary fluctuations.
For instance, fluctuation in testosterone levels when a child is in
distress has been found to be indicative of fathering styles. If a
father's testosterone levels decrease in response to hearing their baby
cry, it is an indication of empathizing with the baby. This is
associated with increased nurturing behavior and better outcomes for the
infant.
Motivation
Testosterone levels play a major role in risk-taking during financial decisions.
Aggression and criminality
Most studies support a link between adult criminality and
testosterone. Nearly all studies of juvenile delinquency and
testosterone are not significant. Most studies have also found
testosterone to be associated with behaviors or personality traits
linked with criminality such as antisocial behavior and alcoholism.
Many studies have also been done on the relationship between more
general aggressive behavior and feelings and testosterone. About half
the studies have found a relationship and about half no relationship. Studies have also found that testosterone facilitates aggression by modulating vasopressin receptors in the hypothalamus.
Testosterone is significantly discussed in relation to aggression
and competitive behavior. There are two theories on the role of
testosterone in aggression and competition. The first one is the challenge hypothesis
which states that testosterone would increase during puberty, thus
facilitating reproductive and competitive behavior which would include
aggression. It is therefore the challenge of competition among males of the species that facilitates aggression and violence.
Studies conducted have found direct correlation between testosterone
and dominance, especially among the most violent criminals in prison who
had the highest testosterone levels.
The same research also found fathers (those outside competitive
environments) had the lowest testosterone levels compared to other
males.
The second theory is similar and is known as "evolutionary neuroandrogenic (ENA) theory of male aggression".
Testosterone and other androgens have evolved to masculinize a brain in
order to be competitive even to the point of risking harm to the person
and others. By doing so, individuals with masculinized brains as a
result of pre-natal and adult life testosterone and androgens enhance
their resource acquiring abilities in order to survive, attract and
copulate with mates as much as possible.
The masculinization of the brain is not just mediated by testosterone
levels at the adult stage, but also testosterone exposure in the womb as
a fetus. Higher pre-natal testosterone indicated by a low digit ratio as well as adult testosterone levels increased risk of fouls or aggression among male players in a soccer game. Studies have also found higher pre-natal testosterone or lower digit ratio to be correlated with higher aggression in males.
The rise in testosterone levels during competition predicted aggression in males but not in females. Subjects who interacted with hand guns and an experimental game showed rise in testosterone and aggression.
Natural selection might have evolved males to be more sensitive to
competitive and status challenge situations and that the interacting
roles of testosterone are the essential ingredient for aggressive
behaviour in these situations.
Testosterone produces aggression by activating subcortical areas in the
brain, which may also be inhibited or suppressed by social norms or
familial situations while still manifesting in diverse intensities and
ways through thoughts, anger, verbal aggression, competition, dominance
and physical violence. Testosterone mediates attraction to cruel and violent cues in men by promoting extended viewing of violent stimuli. Testosterone specific structural brain characteristic can predict aggressive behaviour in individuals.
Testosterone might encourage fair behavior. For one study,
subjects took part in a behavioral experiment where the distribution of a
real amount of money was decided. The rules allowed both fair and
unfair offers. The negotiating partner could subsequently accept or
decline the offer. The fairer the offer, the less probable a refusal by
the negotiating partner. If no agreement was reached, neither party
earned anything. Test subjects with an artificially enhanced
testosterone level generally made better, fairer offers than those who
received placebos, thus reducing the risk of a rejection of their offer
to a minimum. Two later studies have empirically confirmed these
results. However men with high testosterone were significantly 27% less generous in an ultimatum game.
The Annual NY Academy of Sciences has also found anabolic steroid use
(which increases testosterone) to be higher in teenagers, and this was
associated with increased violence. Studies have also found administered testosterone to increase verbal aggression and anger in some participants.
A few studies indicate that the testosterone derivative estradiol (one form of estrogen) might play an important role in male aggression. Estradiol is known to correlate with aggression in male mice. Moreover, the conversion of testosterone to estradiol regulates male aggression in sparrows during breeding season.
Rats who were given anabolic steroids that increase testosterone were
also more physically aggressive to provocation as a result of "threat
sensitivity".
The relationship between testosterone and aggression may also
function indirectly, as it has been proposed that testosterone does not
amplify tendencies towards aggression but rather amplifies whatever
tendencies will allow an individual to maintain social status when
challenged. In most animals, aggression is the means of maintaining
social status. However, humans have multiple ways of obtaining social
status. This could explain why some studies find a link between
testosterone and pro-social behaviour if pro-social behaviour is
rewarded with social status. Thus the link between testosterone and
aggression and violence is due to these being rewarded with social
status.
The relationship may also be one of a "permissive effect" whereby
testosterone does elevate aggression levels but only in the sense of
allowing average aggression levels to be maintained; chemically or
physically castrating the individual will reduce aggression levels
(though it will not eliminate them) but the individual only needs a
small-level of pre-castration testosterone to have aggression levels to
return to normal, which they will remain at even if additional
testosterone is added. Testosterone may also simply exaggerate or
amplify existing aggression; for example, chimpanzees who receive
testosterone increases become more aggressive to chimps lower than them
in the social hierarchy but will still be submissive to chimps higher
than them. Testosterone thus does not make the chimpanzee
indiscriminately aggressive but instead amplifies his pre-existing
aggression towards lower-ranked chimps.
In humans, testosterone appears more to promote status-seeking
and social dominance than simply increasing physical aggression. When
controlling for the effects of belief in having received testosterone,
women who have received testosterone make fairer offers than women who
have not received testosterone.
Brain
The brain is also affected by this sexual differentiation; the enzyme aromatase converts testosterone into estradiol that is responsible for masculinization
of the brain in male mice. In humans, masculinization of the fetal
brain appears, by observation of gender preference in patients with congenital diseases of androgen formation or androgen receptor function, to be associated with functional androgen receptors.
There are some differences between a male and female brain (possibly the result of different testosterone levels), one of them being size: the male human brain is, on average, larger. Men were found to have a total myelinated fiber length of 176 000 km at the age of 20, whereas in women the total length was 149 000 km (approx. 15% less).
No immediate short term effects on mood or behavior were found from the administration of supraphysiologic doses of testosterone for 10 weeks on 43 healthy men. A correlation between testosterone and risk tolerance in career choice exists among women.
Attention, memory, and spatial ability are key cognitive
functions affected by testosterone in humans. Preliminary evidence
suggests that low testosterone levels may be a risk factor for cognitive
decline and possibly for dementia of the Alzheimer's type, a key argument in life extension
medicine for the use of testosterone in anti-aging therapies. Much of
the literature, however, suggests a curvilinear or even quadratic
relationship between spatial performance and circulating testosterone,
where both hypo- and hypersecretion (deficient- and
excessive-secretion) of circulating androgens have negative effects on
cognition.
Immune system and inflammation
Testosterone deficiency is associated with an increased risk of metabolic syndrome, cardiovascular disease and mortality, which are also sequelae of chronic inflammation. Testosterone plasma concentration inversely correlates to multiple biomarkers of inflammation including CRP, interleukin 1 beta, interleukin 6, TNF alpha and endotoxin concentration, as well as leukocyte count. As demonstrated by a meta-analysis, substitution therapy with testosterone results in a significant reduction of inflammatory markers. These effects are mediated by different mechanisms with synergistic action. In androgen-deficient men with concomitant autoimmune thyroiditis, substitution therapy with testosterone leads to a decrease in thyroid autoantibody titres and an increase in thyroid's secretory capacity (SPINA-GT).
Medical use
Testosterone is used as a medication for the treatment of male hypogonadism, gender dysphoria, and certain types of breast cancer. This is known as hormone replacement therapy (HRT) or testosterone replacement therapy (TRT), which maintains serum testosterone levels in the normal range. Decline of testosterone production with age has led to interest in androgen replacement therapy. It is unclear if the use of testosterone for low levels due to aging is beneficial or harmful.
Testosterone is included in the World Health Organization's list of essential medicines, which are the most important medications needed in a basic health system. It is available as a generic medication. It can be administered as a cream or transdermal patch that is applied to the skin, by injection into a muscle, as a tablet that is placed in the cheek, or by ingestion.
Common side effects from testosterone medication include acne, swelling, and breast enlargement in males. Serious side effects may include liver toxicity, heart disease, and behavioral changes. Women and children who are exposed may develop virilization. It is recommended that individuals with prostate cancer not use the medication. It can cause harm if used during pregnancy or breastfeeding.
2020 guidelines from the American College of Physicians support the discussion of testosterone treatment in adult men with age-related low levels of testosterone who have sexual dysfunction.
They recommend yearly evaluation regarding possible improvement and, if
none, to discontinue testosterone; physicians should consider
intramuscular treatments, rather than transdermal treatments, due to
costs and since the effectiveness and harm of either method is similar.
Testosterone treatment for reasons other than possible improvement of
sexual dysfunction may not be recommended.
Biological activity
Steroid hormone activity
The effects of testosterone in humans and other vertebrates occur by way of multiple mechanisms: by activation of the androgen receptor (directly or as dihydrotestosterone), and by conversion to estradiol and activation of certain estrogen receptors. Androgens such as testosterone have also been found to bind to and activate membrane androgen receptors.
Free testosterone (T) is transported into the cytoplasm of target tissue cells, where it can bind to the androgen receptor, or can be reduced to 5α-dihydrotestosterone (DHT) by the cytoplasmic enzyme 5α-reductase.
DHT binds to the same androgen receptor even more strongly than
testosterone, so that its androgenic potency is about 5 times that of T. The T-receptor or DHT-receptor complex undergoes a structural change that allows it to move into the cell nucleus and bind directly to specific nucleotide sequences of the chromosomal DNA. The areas of binding are called hormone response elements (HREs), and influence transcriptional activity of certain genes, producing the androgen effects.
Androgen receptors occur in many different vertebrate body system
tissues, and both males and females respond similarly to similar
levels. Greatly differing amounts of testosterone prenatally, at
puberty, and throughout life account for a share of biological differences between males and females.
The bones and the brain are two important tissues in humans where the primary effect of testosterone is by way of aromatization to estradiol. In the bones, estradiol accelerates ossification of cartilage into bone, leading to closure of the epiphyses
and conclusion of growth. In the central nervous system, testosterone
is aromatized to estradiol. Estradiol rather than testosterone serves as
the most important feedback signal to the hypothalamus (especially
affecting LH secretion). In many mammals, prenatal or perinatal "masculinization" of the sexually dimorphic areas of the brain by estradiol derived from testosterone programs later male sexual behavior.
Neurosteroid activity
Testosterone, via its active metabolite 3α-androstanediol, is a potent positive allosteric modulator of the GABAA receptor.
Testosterone has been found to act as an antagonist of the TrkA and p75NTR, receptors for the neurotrophin nerve growth factor (NGF), with high affinity (around 5 nM). In contrast to testosterone, DHEA and DHEA sulfate have been found to act as high-affinity agonists of these receptors.
Testosterone is an antagonist of the sigma σ1 receptor (Ki = 1,014 or 201 nM).
However, the concentrations of testosterone required for binding the
receptor are far above even total circulating concentrations of
testosterone in adult males (which range between 10 and 35 nM).
Biochemistry
Biosynthesis
Like other steroid hormones, testosterone is derived from cholesterol (see figure). The first step in the biosynthesis involves the oxidative cleavage of the side-chain of cholesterol by cholesterol side-chain cleavage enzyme (P450scc, CYP11A1), a mitochondrial cytochrome P450 oxidase with the loss of six carbon atoms to give pregnenolone. In the next step, two additional carbon atoms are removed by the CYP17A1 (17α-hydroxylase/17,20-lyase) enzyme in the endoplasmic reticulum to yield a variety of C19 steroids.[136] In addition, the 3β-hydroxyl group is oxidized by 3β-hydroxysteroid dehydrogenase to produce androstenedione. In the final and rate limiting step, the C17 keto group androstenedione is reduced by 17β-hydroxysteroid hydrogenase to yield testosterone.
The largest amounts of testosterone (>95%) are produced by the testes in men, while the adrenal glands
account for most of the remainder. Testosterone is also synthesized in
far smaller total quantities in women by the adrenal glands, thecal cells of the ovaries, and, during pregnancy, by the placenta. In the testes, testosterone is produced by the Leydig cells. The male generative glands also contain Sertoli cells, which require testosterone for spermatogenesis.
Like most hormones, testosterone is supplied to target tissues in the
blood where much of it is transported bound to a specific plasma protein, sex hormone-binding globulin (SHBG).
Regulation
Hypothalamic–pituitary–testicular axis
In males, testosterone is synthesized primarily in Leydig cells. The number of Leydig cells in turn is regulated by luteinizing hormone (LH) and follicle-stimulating hormone
(FSH). In addition, the amount of testosterone produced by existing
Leydig cells is under the control of LH, which regulates the expression
of 17β-hydroxysteroid dehydrogenase.
The amount of testosterone synthesized is regulated by the hypothalamic–pituitary–testicular axis (see figure to the right). When testosterone levels are low, gonadotropin-releasing hormone (GnRH) is released by the hypothalamus, which in turn stimulates the pituitary gland
to release FSH and LH. These latter two hormones stimulate the testis
to synthesize testosterone. Finally, increasing levels of testosterone
through a negative feedback loop act on the hypothalamus and pituitary to inhibit the release of GnRH and FSH/LH, respectively.
Factors affecting testosterone levels may include:
- Age: Testosterone levels gradually reduce as men age. This effect is sometimes referred to as andropause or late-onset hypogonadism.
- Exercise: Resistance training increases testosterone levels, however, in older men, that increase can be avoided by protein ingestion. Endurance training in men may lead to lower testosterone levels.
- Nutrients: Vitamin A deficiency may lead to sub-optimal plasma testosterone levels. The secosteroid vitamin D in levels of 400–1000 IU/d (10–25 µg/d) raises testosterone levels. Zinc deficiency lowers testosterone levels but over-supplementation has no effect on serum testosterone. There is limited evidence that low-fat diets may reduce total and free testosterone levels in men.
- Weight loss: Reduction in weight may result in an increase in
testosterone levels. Fat cells synthesize the enzyme aromatase, which
converts testosterone, the male sex hormone, into estradiol, the female
sex hormone. However no clear association between body mass index and testosterone levels has been found.
- Miscellaneous: Sleep: (REM sleep) increases nocturnal testosterone levels. Behavior: Dominance challenges can, in some cases, stimulate increased testosterone release in men. Drugs: Natural or man-made antiandrogens including spearmint tea reduce testosterone levels. Licorice can decrease the production of testosterone and this effect is greater in females.
Distribution
The plasma protein binding of testosterone is 98.0 to 98.5%, with 1.5 to 2.0% free or unbound. It is bound 65% to sex hormone-binding globulin (SHBG) and 33% bound weakly to albumin.
Metabolism
Both testosterone and 5α-DHT are metabolized mainly in the liver. Approximately 50% of testosterone is metabolized via conjugation into testosterone glucuronide and to a lesser extent testosterone sulfate by glucuronosyltransferases and sulfotransferases, respectively. An additional 40% of testosterone is metabolized in equal proportions into the 17-ketosteroids androsterone and etiocholanolone via the combined actions of 5α- and 5β-reductases, 3α-hydroxysteroid dehydrogenase, and 17β-HSD, in that order. Androsterone and etiocholanolone are then glucuronidated and to a lesser extent sulfated similarly to testosterone. The conjugates of testosterone and its hepatic metabolites are released from the liver into circulation and excreted in the urine and bile. Only a small fraction (2%) of testosterone is excreted unchanged in the urine.
In the hepatic 17-ketosteroid pathway of testosterone metabolism,
testosterone is converted in the liver by 5α-reductase and 5β-reductase
into 5α-DHT and the inactive 5β-DHT, respectively. Then, 5α-DHT and 5β-DHT are converted by 3α-HSD into 3α-androstanediol and 3α-etiocholanediol, respectively.
Subsequently, 3α-androstanediol and 3α-etiocholanediol are converted by
17β-HSD into androsterone and etiocholanolone, which is followed by
their conjugation and excretion. 3β-Androstanediol and 3β-etiocholanediol
can also be formed in this pathway when 5α-DHT and 5β-DHT are acted
upon by 3β-HSD instead of 3α-HSD, respectively, and they can then be
transformed into epiandrosterone and epietiocholanolone, respectively. A small portion of approximately 3% of testosterone is reversibly converted in the liver into androstenedione by 17β-HSD.
In addition to conjugation and the 17-ketosteroid pathway, testosterone can also be hydroxylated and oxidized in the liver by cytochrome P450 enzymes, including CYP3A4, CYP3A5, CYP2C9, CYP2C19, and CYP2D6. 6β-Hydroxylation and to a lesser extent 16β-hydroxylation are the major transformations.
The 6β-hydroxylation of testosterone is catalyzed mainly by CYP3A4 and
to a lesser extent CYP3A5 and is responsible for 75 to 80% of cytochrome
P450-mediated testosterone metabolism.
In addition to 6β- and 16β-hydroxytestosterone, 1β-, 2α/β-, 11β-, and
15β-hydroxytestosterone are also formed as minor metabolites.
Certain cytochrome P450 enzymes such as CYP2C9 and CYP2C19 can also
oxidize testosterone at the C17 position to form androstenedione.
Two of the immediate metabolites of testosterone, 5α-DHT and estradiol, are biologically important and can be formed both in the liver and in extrahepatic tissues.
Approximately 5 to 7% of testosterone is converted by 5α-reductase into
5α-DHT, with circulating levels of 5α-DHT about 10% of those of
testosterone, and approximately 0.3% of testosterone is converted into
estradiol by aromatase. 5α-Reductase is highly expressed in the male reproductive organs (including the prostate gland, seminal vesicles, and epididymides), skin, hair follicles, and brain and aromatase is highly expressed in adipose tissue, bone, and the brain. As much as 90% of testosterone is converted into 5α-DHT in so-called androgenic tissues with high 5α-reductase expression, and due to the several-fold greater potency of 5α-DHT as an AR agonist relative to testosterone, it has been estimated that the effects of testosterone are potentiated 2- to 3-fold in such tissues.
Levels
Total levels of testosterone in the body are 264 to 916 ng/dL in men age 19 to 39 years, while mean testosterone levels in adult men have been reported as 630 ng/dL. Levels of testosterone in men decline with age. In women, mean levels of total testosterone have been reported to be 32.6 ng/dL. In women with hyperandrogenism, mean levels of total testosterone have been reported to be 62.1 ng/dL.
Measurement
Testosterone's
bioavailable concentration is commonly determined using the Vermeulen
calculation or more precisely using the modified Vermeulen method, which considers the dimeric form of sex-hormone-binding-globulin.
Both methods use chemical equilibrium to derive the concentration
of bioavailable testosterone: in circulation, testosterone has two
major binding partners, albumin (weakly bound) and
sex-hormone-binding-globulin (strongly bound). These methods are
described in detail in the accompanying figure.
Dimeric sex-hormone-binding-globulin with its testosterone ligands
Two methods for determining the concentration of bioavailable testosterone.
History
Nobel Prize winner,
Leopold Ruzicka of Ciba, a pharmaceutical industry giant that synthesized testosterone.
A testicular
action was linked to circulating blood fractions – now understood to be
a family of androgenic hormones – in the early work on castration and
testicular transplantation in fowl by Arnold Adolph Berthold (1803–1861). Research on the action of testosterone received a brief boost in 1889, when the Harvard professor Charles-Édouard Brown-Séquard
(1817–1894), then in Paris, self-injected subcutaneously a
"rejuvenating elixir" consisting of an extract of dog and guinea pig
testicle. He reported in The Lancet that his vigor and feeling of well-being were markedly restored but the effects were transient,
and Brown-Séquard's hopes for the compound were dashed. Suffering the
ridicule of his colleagues, he abandoned his work on the mechanisms and
effects of androgens in human beings.
In 1927, the University of Chicago's Professor of Physiologic
Chemistry, Fred C. Koch, established easy access to a large source of
bovine testicles — the Chicago stockyards — and recruited students
willing to endure the tedious work of extracting their isolates. In that
year, Koch and his student, Lemuel McGee, derived 20 mg of a substance
from a supply of 40 pounds of bovine testicles that, when administered
to castrated roosters, pigs and rats, re-masculinized them.
The group of Ernst Laqueur at the University of Amsterdam purified
testosterone from bovine testicles in a similar manner in 1934, but the
isolation of the hormone from animal tissues in amounts permitting
serious study in humans was not feasible until three European
pharmaceutical giants—Schering (Berlin, Germany), Organon (Oss, Netherlands) and Ciba (Basel, Switzerland)—began full-scale steroid research and development programs in the 1930s.
The Organon group in the Netherlands were the first to isolate
the hormone, identified in a May 1935 paper "On Crystalline Male Hormone
from Testicles (Testosterone)". They named the hormone testosterone, from the stems of testicle and sterol, and the suffix of ketone. The structure was worked out by Schering's Adolf Butenandt, at the Chemisches Institut of Technical University in Gdańsk.
The chemical synthesis of testosterone from cholesterol was achieved in August that year by Butenandt and Hanisch. Only a week later, the Ciba group in Zurich, Leopold Ruzicka (1887–1976) and A. Wettstein, published their synthesis of testosterone. These independent partial syntheses of testosterone from a cholesterol base earned both Butenandt and Ruzicka the joint 1939 Nobel Prize in Chemistry. Testosterone was identified as 17β-hydroxyandrost-4-en-3-one (C19H28O2),
a solid polycyclic alcohol with a hydroxyl group at the 17th carbon
atom. This also made it obvious that additional modifications on the
synthesized testosterone could be made, i.e., esterification and
alkylation.
The partial synthesis in the 1930s of abundant, potent testosterone esters
permitted the characterization of the hormone's effects, so that
Kochakian and Murlin (1936) were able to show that testosterone raised
nitrogen retention (a mechanism central to anabolism) in the dog, after
which Allan Kenyon's group[196]
was able to demonstrate both anabolic and androgenic effects of
testosterone propionate in eunuchoidal men, boys, and women. The period
of the early 1930s to the 1950s has been called "The Golden Age of
Steroid Chemistry", and work during this period progressed quickly.
Other species
Testosterone is observed in most vertebrates. Testosterone and the classical nuclear androgen receptor first appeared in gnathostomes (jawed vertebrates). Agnathans (jawless vertebrates) such as lampreys do not produce testosterone but instead use androstenedione as a male sex hormone. Fish make a slightly different form called 11-ketotestosterone. Its counterpart in insects is ecdysone. The presence of these ubiquitous steroids in a wide range of animals suggest that sex hormones have an ancient evolutionary history.












