Early explanations of social behavior, such as the lekking of blackcock, spoke of "the good of the species". Blackcocks at the Lek watercolour and bodycolour by Archibald Thorburn, 1901.
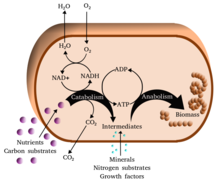
Group selection is a proposed mechanism of evolution in which natural selection acts at the level of the group, instead of at the more conventional level of the individual.
Early authors such as V. C. Wynne-Edwards and Konrad Lorenz
argued that the behavior of animals could affect their survival and
reproduction as groups, speaking for instance of actions for the good of
the species. From the mid 1960s, evolutionary biologists such as John Maynard Smith argued that natural selection acted primarily at the level of the individual. They argued on the basis of mathematical models that individuals would not altruistically sacrifice fitness
for the sake of a group. They persuaded the majority of biologists that
group selection did not occur, other than in special situations such as
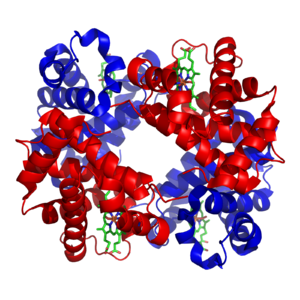
the haplodiploid social insects like honeybees (in the Hymenoptera), where kin selection was possible.
In 1994 David Sloan Wilson and Elliott Sober
argued for multi-level selection, including group selection, on the
grounds that groups, like individuals, could compete. In 2010 three
authors including E. O. Wilson, known for his work on social insects especially ants,
again revisited the arguments for group selection. They argued that
group selection can occur when competition between two or more groups,
some containing altruistic individuals who act cooperatively together,
is more important for survival than competition between individuals
within each group. Their proposals provoked a strong rebuttal from a
large group of evolutionary biologists.
Early Developments
Charles Darwin developed the theory of evolution in his book, Origin of Species. Darwin also made the first suggestion of group selection in The Descent of Man
that the evolution of groups could affect the survival of individuals.
He wrote, "If one man in a tribe... invented a new snare or weapon, the
tribe would increase in number, spread, and supplant other tribes. In a
tribe thus rendered more numerous there would always be a rather better
chance of the birth of other superior and inventive members."
Once Darwinism had been accepted in the modern synthesis
of the mid-twentieth century, animal behavior was glibly explained with
unsubstantiated hypotheses about survival value, which was largely
taken for granted. The naturalist Konrad Lorenz had argued loosely in books like On Aggression (1966) that animal behavior patterns were "for the good of the species", without actually studying survival value in the field. Richard Dawkins noted that Lorenz was a "'good of the species' man" so accustomed to group selection thinking that he did not realize his views "contravened orthodox Darwinian theory". The ethologist Niko Tinbergen
praised Lorenz for his interest in the survival value of behavior, and
naturalists enjoyed Lorenz's writings for the same reason. In 1962, group selection was used as a popular explanation for adaptation by the zoologist V. C. Wynne-Edwards. In 1976, Richard Dawkins wrote a well-known book on the importance of evolution at the level of the gene or the individual, The Selfish Gene.
Social behavior in honeybees is explained by kin selection: their haplodiploid inheritance system makes workers very closely related to their queen (centre).
From the mid 1960s, evolutionary biologists argued that natural selection acted primarily at the level of the individual. In 1964, John Maynard Smith, C. M. Perrins (1964), and George C. Williams in his 1966 book Adaptation and Natural Selection cast serious doubt on group selection as a major mechanism of evolution; Williams's 1971 book Group Selection assembled writings from many authors on the same theme.
It was at that time generally agreed that the primary exception
of social group selection was in the social insects, and the explanation
was limited to the unique inheritance system (involving haplodiploidy) of the eusocial Hymenoptera such as honeybees, which encourages kin selection, since workers are closely related.
Kin selection and inclusive fitness theory
Early group selection models assumed that genes acted independently,
for example a gene that coded for cooperation or altruism.
Genetically-based reproduction of individuals implies that, in group
formation, the altruistic genes would need a way to act for the benefit
of members in the group to enhance the fitness of many individuals with
the same gene.
But it is expected from this model that individuals of the same species
would compete against each other for the same resources. This would put
cooperating individuals at a disadvantage, making genes for cooperation
likely to be eliminated. Group selection on the level of the species is
flawed because it is difficult to see how selective pressures would be
applied to competing/non-cooperating individuals.
Experiments from the late 1970s suggested that selection involving groups was possible. Kin selection
between related individuals is accepted as an explanation of altruistic
behavior. In this model, genetically related individuals cooperate
because survival advantages to one individual also benefit kin who share
some fraction of the same genes, giving a mechanism for favoring
genetic selection.
Inclusive fitness theory, first proposed by W. D. Hamilton
in the early 1960s, gives a selection criterion for evolution of social
traits when social behavior is costly to an individual organism's
survival and reproduction. This behavior could emerge under conditions
such that the statistical likelihood that benefits accrue to the
survival and reproduction of other organisms whom also carry the social
trait. Inclusive fitness theory is a general treatment of the
statistical probabilities of social traits accruing to any other
organisms likely to propagate a copy of the same social trait. Kin selection
theory treats the narrower but simpler case of the benefits to close
genetic relatives (or what biologists call 'kin') who may also carry and
propagate the trait. A significant group of biologists support
inclusive fitness as the explanation for social behavior in a wide range
of species, as supported by experimental data. An article was published
in Nature with over a hundred coauthors.
One of the questions about kin selection is the requirement that
individuals must know if other individuals are related to them, or kin recognition.
Any altruistic act has to preserve similar genes. One argument given by
Hamilton is that many individuals operate in "viscous" conditions, so
that they live in physical proximity to relatives. Under these
conditions, they can act altruistically to any other individual, and it
is likely that the other individual will be related. This population
structure builds a continuum between individual selection, kin
selection, kin group selection and group selection without a clear
boundary for each level. However, early theoretical models by D.S.
Wilson et al. and Taylor
showed that pure population viscosity cannot lead to cooperation and
altruism. This is because any benefit generated by kin cooperation is
exactly cancelled out by kin competition; additional offspring from
cooperation are eliminated by local competition. Mitteldorf and D. S.
Wilson later showed that if the population is allowed to fluctuate, then
local populations can temporarily store the benefit of local
cooperation and promote the evolution of cooperation and altruism.
By assuming individual differences in adaptations, Yang further showed
that the benefit of local altruism can be stored in the form of
offspring quality and thus promote the evolution of altruism even if the
population does not fluctuate. This is because local competition among
more individuals resulting from local altruism increases the average
local fitness of the individuals that survive.
Another explanation for the recognition of genes for altruism is
that a single trait, group reciprocal kindness, is capable of explaining
the vast majority of altruism that is generally accepted as "good" by
modern societies. The phenotype of altruism relies on recognition of the
altruistic behavior by itself. The trait of kindness will be recognized
by sufficiently intelligent and undeceived organisms in other
individuals with the same trait. Moreover, the existence of such a trait
predicts a tendency for kindness to unrelated organisms that are
apparently kind, even if the organisms are of a completely different
species. The gene need not be exactly the same, so long as the effect or
phenotype is similar. Multiple versions of the gene—or even meme—would have virtually the same effect. This explanation was given by Richard Dawkins as an analogy of a man with a green beard.
Green-bearded men are imagined as tending to cooperate with each other
simply by seeing a green beard, where the green beard trait is
incidentally linked to the reciprocal kindness trait.
Multilevel selection theory
Kin selection or inclusive fitness is accepted as an explanation for
cooperative behavior in many species, but there are some species,
including some human behavior, that are difficult to explain with only
this approach. In particular, it does not seem to explain the rapid rise
of human civilization. David Sloan Wilson has argued that other factors must also be considered in evolution. Wilson and others have continued to develop group selection models.
Early group selection models were flawed because they assumed
that genes acted independently; but genetically-based interactions among
individuals are ubiquitous in group formation because genes must
cooperate for the benefit of association in groups to enhance the
fitness of group members.
Additionally, group selection on the level of the species is flawed
because it is difficult to see how selective pressures would be applied;
selection in social species of groups against other groups, rather than
the species entire, seems to be the level at which selective pressures
are plausible. On the other hand, kin selection is accepted as an
explanation of altruistic behavior.
Some biologists argue that kin selection and multilevel selection are
both needed to "obtain a complete understanding of the evolution of a
social behavior system".
In 1994 David Sloan Wilson and Elliott Sober
argued that the case against group selection had been overstated. They
considered whether groups can have functional organization in the same
way as individuals, and consequently whether groups can be "vehicles"
for selection.
They do not posit evolution on the level of the species, but selective
pressures that winnow out small groups within a species, e.g. groups of
social insects or primates. Groups that cooperate better might survive
and reproduce more than those that did not. Resurrected in this way,
Wilson & Sober's new group selection is called multilevel selection
theory.
In 2010, M. A. Nowak, C. E. Tarnita and E. O. Wilson argued for multi-level selection, including group selection, to correct what they saw as deficits in the explanatory power of inclusive fitness.
The response was a back-lash from 137 other evolutionary biologists who
argued "that their arguments are based upon a misunderstanding of
evolutionary theory and a misrepresentation of the empirical
literature".
David Sloan Wilson and Elliott Sober's 1994 Multilevel Selection Model, illustrated by a nested set of Russian matryoshka dolls. Wilson himself compared his model to such a set.
Wilson compared the layers of competition and evolution to nested sets of Russian matryoshka dolls. The lowest level is the genes, next come the cells, then the organism
level and finally the groups. The different levels function cohesively
to maximize fitness, or reproductive success. The theory asserts that
selection for the group level, involving competition between groups,
must outweigh the individual level, involving individuals competing
within a group, for a group-benefiting trait to spread.
Multilevel selection theory focuses on the phenotype because it looks at the levels that selection directly acts upon.
For humans, social norms can be argued to reduce individual level
variation and competition, thus shifting selection to the group level.
The assumption is that variation between different groups is larger than
variation within groups. Competition and selection can operate at all
levels regardless of scale. Wilson wrote, "At all scales, there must be
mechanisms that coordinate the right kinds of action and prevent
disruptive forms of self-serving behavior at lower levels of social
organization."
E. O. Wilson summarized, "In a group, selfish individuals beat
altruistic individuals. But, groups of altruistic individuals beat
groups of selfish individuals."
Wilson ties the multilevel selection theory regarding humans to another theory, gene-culture coevolution,
by acknowledging that culture seems to characterize a group-level
mechanism for human groups to adapt to environmental changes.
MLS theory can be used to evaluate the balance between group selection and individual selection in specific cases.
An experiment by William Muir compared egg productivity in hens,
showing that a hyper-aggressive strain had been produced through
individual selection, leading to many fatal attacks after only six
generations; by implication, it could be argued that group selection
must have been acting to prevent this in real life. Group selection has most often been postulated in humans and, notably, eusocial Hymenoptera that make cooperation a driving force of their adaptations over time and have a unique system of inheritance involving haplodiploidy that allows the colony to function as an individual while only the queen reproduces.
Wilson and Sober's work revived interest in multilevel selection. In a 2005 article, E. O. Wilson
argued that kin selection could no longer be thought of as underlying
the evolution of extreme sociality, for two reasons. First, he
suggested, the argument that haplodiploid
inheritance (as in the Hymenoptera) creates a strong selection pressure
towards nonreproductive castes is mathematically flawed. Second, eusociality
no longer seems to be confined to the hymenopterans; increasing numbers
of highly social taxa have been found in the years since Wilson's
foundational text Sociobiology: A New Synthesis was published in 1975. These including a variety of insect species, as well as two rodent species (the naked mole-rat and the Damaraland mole rat). Wilson suggests the equation for Hamilton's rule:
-
- rb > c
(where b represents the benefit to the recipient of altruism, c the
cost to the altruist, and r their degree of relatedness) should be
replaced by the more general equation
-
- rbk + be > c
in which bk is the benefit to kin (b in the original equation) and be
is the benefit accruing to the group as a whole. He then argues that,
in the present state of the evidence in relation to social insects, it
appears that be>rbk, so that altruism needs to
be explained in terms of selection at the colony level rather than at
the kin level. However, kin selection and group selection are not
distinct processes, and the effects of multi-level selection are already
accounted for in Hamilton's rule, rb>c, provided that an expanded definition of r, not requiring Hamilton's original assumption of direct genealogical relatedness, is used, as proposed by E. O. Wilson himself.
Spatial populations of predators and prey show restraint of
reproduction at equilibrium, both individually and through social
communication, as originally proposed by Wynne-Edwards. While these
spatial populations do not have well-defined groups for group selection,
the local spatial interactions of organisms in transient groups are
sufficient to lead to a kind of multi-level selection. There is however
as yet no evidence that these processes operate in the situations where
Wynne-Edwards posited them.
Rauch et al.'s analysis of host-parasite
evolution, which even E. O. Wilson recognised as a situation where
group selection was possible (1975), is broadly hostile to group
selection. Specifically, the parasites do not individually moderate
their transmission; rather, more transmissible variants "continually
arise and grow rapidly for many generations but eventually go extinct
before dominating the system."
Applications
Differing evolutionarily stable strategies
The
problem with group selection is that for a whole group to get a single
trait, it must spread through the whole group first by regular evolution. But, as J. L. Mackie suggested, when there are many different groups, each with a different evolutionarily stable strategy, there is selection between the different strategies, since some are worse than others.
For example, a group where altruism was universal would indeed
outcompete a group where every creature acted in its own interest, so
group selection might seem feasible; but a mixed group of altruists and
non-altruists would be vulnerable to cheating by non-altruists within
the group, so group selection would collapse.
Implications in population biology
Social behaviors such as altruism and group relationships can impact many aspects of population dynamics, such as intraspecific competition and interspecific
interactions. In 1871, Darwin argued that group selection occurs when
the benefits of cooperation or altruism between subpopulations are
greater than the individual benefits of egotism within a subpopulation.
This supports the idea of multilevel selection, but kinship also plays
an integral role because many subpopulations are composed of closely
related individuals. An example of this can be found in lions, which are
simultaneously cooperative and territorial.
Within a pride, males protect the pride from outside males, and
females, who are commonly sisters, communally raise cubs and hunt.
However, this cooperation seems to be density dependent. When resources
are limited, group selection favors prides that work together to hunt.
When prey is abundant, cooperation is no longer beneficial enough to
outweigh the disadvantages of altruism, and hunting is no longer
cooperative.
Interactions between different species can also be affected by
multilevel selection. Predator-prey relationships can also be affected.
Individuals of certain monkey species howl to warn the group of the
approach of a predator.
The evolution of this trait benefits the group by providing protection,
but could be disadvantageous to the individual if the howling draws the
predator's attention to them. By affecting these interspecific
interactions, multilevel and kinship selection can change the population
dynamics of an ecosystem.
Multilevel selection attempts to explain the evolution of altruistic behavior in terms of quantitative genetics. Increased frequency or fixation
of altruistic alleles can be accomplished through kin selection, in
which individuals engage in altruistic behavior to promote the fitness
of genetically similar individuals such as siblings. However, this can
lead to inbreeding depression,
which typically lowers the overall fitness of a population. However, if
altruism were to be selected for through an emphasis on benefit to the
group as opposed to relatedness and benefit to kin, both the altruistic
trait and genetic diversity could be preserved. However, relatedness
should still remain a key consideration in studies of multilevel
selection. Experimentally imposed multilevel selection on Japanese quail
was more effective by an order of magnitude on closely related kin
groups than on randomized groups of individuals.
Gene-culture coevolution in humans
Humanity has developed extremely rapidly, arguably through gene-culture coevolution, leading to complex cultural artefacts like the gopuram of the Sri Mariammam temple, Singapore.
Gene-culture coevolution
(also called dual inheritance theory) is a modern hypothesis
(applicable mostly to humans) that combines evolutionary biology and
modern sociobiology to indicate group selection.
It treats culture as a separate evolutionary system that acts in
parallel to the usual genetic evolution to transform human traits.
It is believed that this approach of combining genetic influence with
cultural influence over several generations is not present in the other
hypotheses such as reciprocal altruism and kin selection, making
gene-culture evolution one of the strongest realistic hypotheses for
group selection. Fehr provides evidence of group selection taking place
in humans presently with experimentation through logic games such as
prisoner’s dilemma, the type of thinking that humans have developed many
generations ago.
Gene-culture coevolution allows humans to develop highly distinct
adaptations to the local pressures and environments more quickly than
with genetic evolution alone. Robert Boyd and Peter J. Richerson,
two strong proponents of cultural evolution, postulate that the act of
social learning, or learning in a group as done in group selection,
allows human populations to accrue information over many generations.
This leads to cultural evolution of behaviors and technology alongside
genetic evolution. Boyd and Richerson believe that the ability to
collaborate evolved during the Middle Pleistocene, a million years ago, in response to a rapidly changing climate.
In 2003, the behavioral scientist Herbert Gintis
examined cultural evolution statistically, offering evidence that
societies that promote pro-social norms have higher survival rates than
societies that do not.
Gintis wrote that genetic and cultural evolution can work together.
Genes transfer information in DNA, and cultures transfer information
encoded in brains, artifacts, or documents. Language, tools, lethal
weapons, fire, cooking, etc., have a long-term effect on genetics. For
example, cooking led to a reduction of size of the human gut, since less
digestion is needed for cooked food. Language led to a change in the
human larynx and an increase in brain size. Projectile weapons led to
changes in human hands and shoulders, such that humans are much better
at throwing objects than the closest human relative, the chimpanzee.
Criticism
The use of the Price equation to support group selection was challenged by van Veelen in 2012, arguing that it is based on invalid mathematical assumptions.
Richard Dawkins and other advocates of the gene-centered view of evolution remain unconvinced about group selection. In particular, Dawkins suggests that group selection fails to make an appropriate distinction between replicators and vehicles.
The psychologist Steven Pinker
concluded that "group selection has no useful role to play in
psychology or social science", since it "is not a precise implementation
of the theory of natural selection, as it is, say, in genetic algorithms or artificial life simulations. Instead it is a loose metaphor, more like the struggle among kinds of tires or telephones."
The evolutionary biologist Jerry Coyne summarized the arguments in The New York Times in non-technical terms as follows:
Group selection isn't widely accepted by evolutionists for several reasons. First, it's not an efficient way to select for traits, like altruistic behavior, that are supposed to be detrimental to the individual but good for the group. Groups divide to form other groups much less often than organisms reproduce to form other organisms, so group selection for altruism would be unlikely to override the tendency of each group to quickly lose its altruists through natural selection favoring cheaters. Further, little evidence exists that selection on groups has promoted the evolution of any trait. Finally, other, more plausible evolutionary forces, like direct selection on individuals for reciprocal support, could have made humans prosocial. These reasons explain why only a few biologists, like [David Sloan] Wilson and E. O. Wilson (no relation), advocate group selection as the evolutionary source of cooperation.