From Wikipedia, the free encyclopedia

DNA damage resulting in multiple broken chromosomes
DNA repair is a collection of processes by which a
cell identifies and corrects damage to the
DNA molecules that encode its
genome. In human cells, both normal
metabolic activities and environmental factors such as
radiation can cause DNA damage, resulting in as many as 1
million individual
molecular lesions per cell per day. Many of these lesions cause structural damage to the DNA molecule and can alter or eliminate the cell's ability to
transcribe the
gene that the affected DNA encodes. Other lesions induce potentially harmful
mutations in the cell's genome, which affect the survival of its daughter cells after it undergoes
mitosis.
As a consequence, the DNA repair process is constantly active as it
responds to damage in the DNA structure. When normal repair processes
fail, and when cellular
apoptosis does not occur, irreparable DNA damage may occur, including double-strand breaks and
DNA crosslinkages (interstrand crosslinks or ICLs). This can eventually lead to malignant tumors, or
cancer as per the
two hit hypothesis.
The rate of DNA repair is dependent on many factors, including
the cell type, the age of the cell, and the extracellular environment. A
cell that has accumulated a large amount of DNA damage, or one that no
longer effectively repairs damage incurred to its DNA, can enter one of
three possible states:
- an irreversible state of dormancy, known as senescence
- cell suicide, also known as apoptosis or programmed cell death
- unregulated cell division, which can lead to the formation of a tumor that is cancerous
The DNA repair ability of a cell is vital to the integrity of its
genome and thus to the normal functionality of that organism. Many genes
that were initially shown to influence
life span have turned out to be involved in DNA damage repair and protection.
Paul Modrich talks about himself and his work in DNA repair.
DNA damage
DNA damage, due to environmental factors and normal
metabolic processes inside the cell, occurs at a rate of 10,000 to 1,000,000 molecular lesions per cell per day.
While this constitutes only 0.000165% of the human genome's
approximately 6 billion bases (3 billion base pairs), unrepaired lesions
in critical genes (such as
tumor suppressor genes) can impede a cell's ability to carry out its function and appreciably increase the likelihood of
tumor formation and contribute to
tumour heterogeneity.
The vast majority of DNA damage affects the
primary structure
of the double helix; that is, the bases themselves are chemically
modified. These modifications can in turn disrupt the molecules' regular
helical structure by introducing non-native chemical bonds or bulky
adducts that do not fit in the standard double helix. Unlike
proteins and
RNA, DNA usually lacks
tertiary structure and therefore damage or disturbance does not occur at that level. DNA is, however,
supercoiled and wound around "packaging" proteins called
histones (in eukaryotes), and both superstructures are vulnerable to the effects of DNA damage.
Sources
DNA damage can be subdivided into two main types:
- endogenous damage such as attack by reactive oxygen species produced from normal metabolic byproducts (spontaneous mutation), especially the process of oxidative deamination
- also includes replication errors
- exogenous damage caused by external agents such as
- ultraviolet [UV 200–400 nm] radiation from the sun or other artificial light sources
- other radiation frequencies, including x-rays and gamma rays
- hydrolysis or thermal disruption
- certain plant toxins
- human-made mutagenic chemicals, especially aromatic compounds that act as DNA intercalating agents
- viruses
The replication of damaged DNA before cell division can lead to the
incorporation of wrong bases opposite damaged ones. Daughter cells that
inherit these wrong bases carry mutations from which the original DNA
sequence is unrecoverable (except in the rare case of a
back mutation, for example, through
gene conversion).
Types
There are several types of damage to DNA due to endogenous cellular processes:
- oxidation of bases [e.g. 8-oxo-7,8-dihydroguanine (8-oxoG)] and generation of DNA strand interruptions from reactive oxygen species,
- alkylation of bases (usually methylation), such as formation of 7-methylguanosine, 1-methyladenine, 6-O-Methylguanine
- hydrolysis of bases, such as deamination, depurination, and depyrimidination.
- "bulky adduct formation" (e.g., benzo[a]pyrene diol epoxide-dG adduct, aristolactam I-dA adduct)
- mismatch of bases, due to errors in DNA replication,
in which the wrong DNA base is stitched into place in a newly forming
DNA strand, or a DNA base is skipped over or mistakenly inserted.
- Monoadduct damage cause by change in single nitrogenous base of DNA
- Diadduct damage
Damage caused by exogenous agents comes in many forms. Some examples are:
- UV-B light causes crosslinking between adjacent cytosine and thymine bases creating pyrimidine dimers. This is called direct DNA damage.
- UV-A light creates mostly free radicals. The damage caused by free radicals is called indirect DNA damage.
- Ionizing radiation such as that created by radioactive decay or in cosmic rays
causes breaks in DNA strands. Intermediate-level ionizing radiation
may induce irreparable DNA damage (leading to replicational and
transcriptional errors needed for neoplasia or may trigger viral
interactions) leading to pre-mature aging and cancer.
- Thermal disruption at elevated temperature increases the rate of depurination (loss of purine bases from the DNA backbone) and single-strand breaks. For example, hydrolytic depurination is seen in the thermophilic bacteria, which grow in hot springs at 40–80 °C. The rate of depurination (300 purine
residues per genome per generation) is too high in these species to be
repaired by normal repair machinery, hence a possibility of an adaptive response cannot be ruled out.
- Industrial chemicals such as vinyl chloride and hydrogen peroxide, and environmental chemicals such as polycyclic aromatic hydrocarbons
found in smoke, soot and tar create a huge diversity of DNA adducts-
ethenobases, oxidized bases, alkylated phosphotriesters and crosslinking of DNA, just to name a few.
UV damage, alkylation/methylation, X-ray damage and oxidative damage
are examples of induced damage. Spontaneous damage can include the loss
of a base, deamination, sugar
ring puckering
and tautomeric shift. Constitutive (spontaneous) DNA damage caused by
endogenous oxidants can be detected as a low level of histone H2AX
phosphorylation in untreated cells.
Nuclear versus mitochondrial
In human cells, and
eukaryotic cells in general, DNA is found in two cellular locations – inside the
nucleus and inside the
mitochondria. Nuclear DNA (nDNA) exists as
chromatin during non-replicative stages of the
cell cycle and is condensed into aggregate structures known as
chromosomes during
cell division. In either state the DNA is highly compacted and wound up around bead-like proteins called
histones.
Whenever a cell needs to express the genetic information encoded in its
nDNA the required chromosomal region is unravelled, genes located
therein are expressed, and then the region is condensed back to its
resting conformation. Mitochondrial DNA (mtDNA) is located inside
mitochondria
organelles,
exists in multiple copies, and is also tightly associated with a number
of proteins to form a complex known as the nucleoid. Inside
mitochondria,
reactive oxygen species (ROS), or
free radicals, byproducts of the constant production of
adenosine triphosphate (ATP) via
oxidative phosphorylation,
create a highly oxidative environment that is known to damage mtDNA. A
critical enzyme in counteracting the toxicity of these species is
superoxide dismutase, which is present in both the mitochondria and
cytoplasm of eukaryotic cells.
Senescence and apoptosis
Senescence, an irreversible process in which the cell no longer
divides, is a protective response to the shortening of the
chromosome ends. The telomeres are long regions of repetitive
noncoding DNA that cap chromosomes and undergo partial degradation each time a cell undergoes division (see
Hayflick limit). In contrast,
quiescence is a reversible state of cellular dormancy that is unrelated to genome damage.
Senescence in cells may serve as a functional alternative to apoptosis
in cases where the physical presence of a cell for spatial reasons is
required by the organism,
which serves as a "last resort" mechanism to prevent a cell with
damaged DNA from replicating inappropriately in the absence of
pro-growth
cellular signaling. Unregulated cell division can lead to the formation of a tumor,
which is potentially lethal to an organism. Therefore, the induction of
senescence and apoptosis is considered to be part of a strategy of
protection against cancer.
Mutation
It is
important to distinguish between DNA damage and mutation, the two major
types of error in DNA. DNA damage and mutation are fundamentally
different. Damage results in physical abnormalities in the DNA, such as
single- and double-strand breaks,
8-hydroxydeoxyguanosine
residues, and polycyclic aromatic hydrocarbon adducts. DNA damage can
be recognized by enzymes, and thus can be correctly repaired if
redundant information, such as the undamaged sequence in the
complementary DNA strand or in a homologous chromosome, is available for
copying. If a cell retains DNA damage, transcription of a gene can be
prevented, and thus translation into a protein will also be blocked.
Replication may also be blocked or the cell may die.
In contrast to DNA damage, a mutation is a change in the base
sequence of the DNA. A mutation cannot be recognized by enzymes once the
base change is present in both DNA strands, and thus a mutation cannot
be repaired. At the cellular level, mutations can cause alterations in
protein function and regulation. Mutations are replicated when the cell
replicates. In a population of cells, mutant cells will increase or
decrease in frequency according to the effects of the mutation on the
ability of the cell to survive and reproduce.
Although distinctly different from each other, DNA damage and
mutation are related because DNA damage often causes errors of DNA
synthesis during replication or repair; these errors are a major source
of mutation.
Given these properties of DNA damage and mutation, it can be seen
that DNA damage is a special problem in non-dividing or slowly-dividing
cells, where unrepaired damage will tend to accumulate over time. On
the other hand, in rapidly-dividing cells, unrepaired DNA damage that
does not kill the cell by blocking replication will tend to cause
replication errors and thus mutation. The great majority of mutations
that are not neutral in their effect are deleterious to a cell's
survival. Thus, in a population of cells composing a tissue with
replicating cells, mutant cells will tend to be lost. However,
infrequent mutations that provide a survival advantage will tend to
clonally expand at the expense of neighboring cells in the tissue. This
advantage to the cell is disadvantageous to the whole organism, because
such mutant cells can give rise to cancer. Thus, DNA damage in
frequently dividing cells, because it gives rise to mutations, is a
prominent cause of cancer. In contrast, DNA damage in
infrequently-dividing cells is likely a prominent cause of aging.
Mechanisms
Cells cannot function if DNA damage corrupts the integrity and accessibility of essential information in the
genome
(but cells remain superficially functional when non-essential genes are
missing or damaged). Depending on the type of damage inflicted on the
DNA's double helical structure, a variety of repair strategies have
evolved to restore lost information. If possible, cells use the
unmodified complementary strand of the DNA or the sister
chromatid
as a template to recover the original information. Without access to a
template, cells use an error-prone recovery mechanism known as
translesion synthesis as a last resort.
Damage to DNA alters the spatial configuration of the helix, and
such alterations can be detected by the cell. Once damage is localized,
specific DNA repair molecules bind at or near the site of damage,
inducing other molecules to bind and form a complex that enables the
actual repair to take place.
Direct reversal
Cells
are known to eliminate three types of damage to their DNA by chemically
reversing it. These mechanisms do not require a template, since the
types of damage they counteract can occur in only one of the four bases.
Such direct reversal mechanisms are specific to the type of damage
incurred and do not involve breakage of the phosphodiester backbone. The
formation of
pyrimidine dimers upon irradiation with UV light results in an abnormal covalent bond between adjacent pyrimidine bases. The
photoreactivation process directly reverses this damage by the action of the enzyme
photolyase, whose activation is obligately dependent on energy absorbed from
blue/UV light (300–500 nm
wavelength) to promote catalysis. Photolyase, an old enzyme present in
bacteria,
fungi, and most
animals no longer functions in humans, who instead use
nucleotide excision repair
to repair damage from UV irradiation. Another type of damage,
methylation of guanine bases, is directly reversed by the protein methyl
guanine methyl transferase (MGMT), the bacterial equivalent of which is
called
ogt. This is an expensive process because each MGMT molecule can be used only once; that is, the reaction is
stoichiometric rather than
catalytic. A generalized response to methylating agents in bacteria is known as the
adaptive response and confers a level of resistance to alkylating agents upon sustained exposure by upregulation of alkylation repair enzymes. The third type of DNA damage reversed by cells is certain methylation of the bases cytosine and adenine.
Single-strand damage
Structure of the base-excision repair enzyme
uracil-DNA glycosylase excising a hydrolytically-produced uracil residue from DNA. The uracil residue is shown in yellow.
When only one of the two strands of a double helix has a defect, the
other strand can be used as a template to guide the correction of the
damaged strand. In order to repair damage to one of the two paired
molecules of DNA, there exist a number of
excision repair
mechanisms that remove the damaged nucleotide and replace it with an
undamaged nucleotide complementary to that found in the undamaged DNA
strand.
- Base excision repair
(BER): damaged single bases or nucleotides are most commonly repaired
by removing the base or the nucleotide involved and then inserting the
correct base or nucleotide. In base excision repair, a glycosylase
enzyme removes the damaged base from the DNA by cleaving the bond
between the base and the deoxyribose. These enzymes remove a single base
to create an apurinic or apyrimidinic site (AP site). Enzymes called AP endonucleases nick
the damaged DNA backbone at the AP site. DNA polymerase then removes
the damaged region using its 5’ to 3’ exonuclease activity and
correctly synthesizes the new strand using the complementary strand as a
template. The gap is then sealed by enzyme DNA ligase.
- Nucleotide excision repair (NER): bulky, helix-distorting damage, such as pyrimidine dimerization
caused by UV light is usually repaired by a three-step process. First
the damage is recognized, then 12-24 nucleotide-long strands of DNA are
removed both upstream and downstream of the damage site by endonucleases, and the removed DNA region is then resynthesized. NER is a highly evolutionarily conserved repair mechanism and is used in nearly all eukaryotic and prokaryotic cells. In prokaryotes, NER is mediated by Uvr proteins. In eukaryotes, many more proteins are involved, although the general strategy is the same.
- Mismatch repair systems are present in essentially all cells to correct errors that are not corrected by proofreading.
These systems consist of at least two proteins. One detects the
mismatch, and the other recruits an endonuclease that cleaves the newly
synthesized DNA strand close to the region of damage. In E. coli ,
the proteins involved are the Mut class proteins: MutS, MutL, and MutH.
In most Eukaryotes, the analog for MutS is MSH and the analog for MutL
is MLH. MutH is only present in bacteria. This is followed by removal of
damaged region by an exonuclease, resynthesis by DNA polymerase, and
nick sealing by DNA ligase.
Double-strand breaks
Double-strand break repair pathway models
Double-strand breaks, in which both strands in the double helix are
severed, are particularly hazardous to the cell because they can lead to
genome rearrangements. In fact, when a double-strand break is
accompanied by a cross-linkage joining the two strands at the same
point, neither strand can be used as a template for the repair
mechanisms, so that the cell will not be able to complete mitosis when
it next divides, and will either die or, in rare cases, undergo a
mutation. Three mechanisms exist to repair double-strand breaks (DSBs):
non-homologous end joining (NHEJ),
microhomology-mediated end joining (MMEJ), and
homologous recombination (HR). In an
in vitro system, MMEJ occurred in mammalian cells at the levels of 10–20% of HR when both HR and NHEJ mechanisms were also available.
DNA
ligase, shown above repairing chromosomal damage, is an enzyme that
joins broken nucleotides together by catalyzing the formation of an
internucleotide
ester bond between the phosphate backbone and the deoxyribose nucleotides.
In NHEJ,
DNA Ligase IV, a specialized
DNA ligase that forms a complex with the cofactor
XRCC4, directly joins the two ends.
To guide accurate repair, NHEJ relies on short homologous sequences
called microhomologies present on the single-stranded tails of the DNA
ends to be joined. If these overhangs are compatible, repair is usually
accurate.
NHEJ can also introduce mutations during repair. Loss of damaged
nucleotides at the break site can lead to deletions, and joining of
nonmatching termini forms insertions or translocations. NHEJ is
especially important before the cell has replicated its DNA, since there
is no template available for repair by homologous recombination. There
are "backup" NHEJ pathways in higher
eukaryotes. Besides its role as a genome caretaker, NHEJ is required for joining hairpin-capped double-strand breaks induced during
V(D)J recombination, the process that generates diversity in
B-cell and
T-cell receptors in the
vertebrate immune system.
Homologous recombination requires the presence of an identical or
nearly identical sequence to be used as a template for repair of the
break. The enzymatic machinery responsible for this repair process is
nearly identical to the machinery responsible for
chromosomal crossover during meiosis. This pathway allows a damaged chromosome to be repaired using a sister
chromatid (available in G2 after DNA replication) or a
homologous chromosome
as a template. DSBs caused by the replication machinery attempting to
synthesize across a single-strand break or unrepaired lesion cause
collapse of the
replication fork and are typically repaired by recombination.
MMEJ starts with short-range
end resection by
MRE11 nuclease on either side of a double-strand break to reveal microhomology regions. In further steps,
Poly (ADP-ribose) polymerase 1 (PARP1) is required and may be an early step in MMEJ. There is pairing of microhomology regions followed by recruitment of
flap structure-specific endonuclease 1 (FEN1) to remove overhanging flaps. This is followed by recruitment of
XRCC1–
LIG3
to the site for ligating the DNA ends, leading to an intact DNA. MMEJ
is always accompanied by a deletion, so that MMEJ is a mutagenic pathway
for DNA repair.
Topoisomerases introduce both single- and double-strand breaks in the course of changing the DNA's state of
supercoiling,
which is especially common in regions near an open replication fork.
Such breaks are not considered DNA damage because they are a natural
intermediate in the topoisomerase biochemical mechanism and are
immediately repaired by the enzymes that created them.
Translesion synthesis
Translesion synthesis (TLS) is a DNA damage tolerance process that allows the
DNA replication machinery to replicate past DNA lesions such as
thymine dimers or
AP sites. It involves switching out regular
DNA polymerases
for specialized translesion polymerases (i.e. DNA polymerase IV or V,
from the Y Polymerase family), often with larger active sites that can
facilitate the insertion of bases opposite damaged nucleotides. The
polymerase switching is thought to be mediated by, among other factors,
the post-translational modification of the replication
processivity factor
PCNA.
Translesion synthesis polymerases often have low fidelity (high
propensity to insert wrong bases) on undamaged templates relative to
regular polymerases. However, many are extremely efficient at inserting
correct bases opposite specific types of damage. For example,
Pol η mediates error-free bypass of lesions induced by
UV irradiation, whereas
Pol ι introduces mutations at these sites. Pol η is known to add the first adenine across the
T^T photodimer using
Watson-Crick base pairing and the second adenine will be added in its syn conformation using
Hoogsteen base pairing. From a cellular perspective, risking the introduction of
point mutations
during translesion synthesis may be preferable to resorting to more
drastic mechanisms of DNA repair, which may cause gross chromosomal
aberrations or cell death. In short, the process involves specialized
polymerases
either bypassing or repairing lesions at locations of stalled DNA
replication. For example, Human DNA polymerase eta can bypass complex
DNA lesions like guanine-thymine intra-strand crosslink, G[8,5-Me]T,
although it can cause targeted and semi-targeted mutations. Paromita Raychaudhury and Ashis Basu studied the toxicity and mutagenesis of the same lesion in
Escherichia coli by replicating a G[8,5-Me]T-modified plasmid in
E. coli
with specific DNA polymerase knockouts. Viability was very low in a
strain lacking pol II, pol IV, and pol V, the three SOS-inducible DNA
polymerases, indicating that translesion synthesis is conducted
primarily by these specialized DNA polymerases.
A bypass platform is provided to these polymerases by
Proliferating cell nuclear antigen (PCNA). Under normal circumstances, PCNA bound to polymerases replicates the DNA. At a site of
lesion, PCNA is ubiquitinated, or modified, by the RAD6/
RAD18 proteins to provide a platform for the specialized polymerases to bypass the lesion and resume DNA replication.
After translesion synthesis, extension is required. This extension can
be carried out by a replicative polymerase if the TLS is error-free, as
in the case of Pol η, yet if TLS results in a mismatch, a specialized
polymerase is needed to extend it;
Pol ζ.
Pol ζ is unique in that it can extend terminal mismatches, whereas more
processive polymerases cannot. So when a lesion is encountered, the
replication fork will stall, PCNA will switch from a processive
polymerase to a TLS polymerase such as Pol ι to fix the lesion, then
PCNA may switch to Pol ζ to extend the mismatch, and last PCNA will
switch to the processive polymerase to continue replication.
Global response to DNA damage
Cells exposed to
ionizing radiation,
ultraviolet light
or chemicals are prone to acquire multiple sites of bulky DNA lesions
and double-strand breaks. Moreover, DNA damaging agents can damage other
biomolecules such as
proteins,
carbohydrates,
lipids, and
RNA. The accumulation of damage, to be specific, double-strand breaks or adducts stalling the
replication forks, are among known stimulation signals for a global response to DNA damage.
The global response to damage is an act directed toward the cells' own
preservation and triggers multiple pathways of macromolecular repair,
lesion bypass, tolerance, or
apoptosis. The common features of global response are induction of multiple
genes,
cell cycle arrest, and inhibition of
cell division.
Initial steps
The packaging of eukaryotic DNA into
chromatin
presents a barrier to all DNA-based processes that require recruitment
of enzymes to their sites of action. To allow DNA repair, the chromatin
must be
remodeled. In eukaryotes,
ATP dependent
chromatin remodeling complexes and
histone-modifying enzymes are two predominant factors employed to accomplish this remodeling process.
Chromatin relaxation occurs rapidly at the site of a DNA damage. In one of the earliest steps, the stress-activated protein kinase,
c-Jun N-terminal kinase (JNK), phosphorylates
SIRT6 on serine 10 in response to double-strand breaks or other DNA damage. This
post-translational modification
facilitates the mobilization of SIRT6 to DNA damage sites, and is
required for efficient recruitment of poly (ADP-ribose) polymerase 1
(PARP1) to DNA break sites and for efficient repair of DSBs.
PARP1
protein starts to appear at DNA damage sites in less than a second,
with half maximum accumulation within 1.6 seconds after the damage
occurs. PARP1 synthesizes
polymeric adenosine diphosphate ribose (poly (ADP-ribose) or PAR) chains on itself. Next the chromatin remodeler
ALC1
quickly attaches to the product of PARP1 action, a poly-ADP ribose
chain, and ALC1 completes arrival at the DNA damage within 10 seconds of
the occurrence of the damage. About half of the maximum chromatin relaxation, presumably due to action of ALC1, occurs by 10 seconds. This then allows recruitment of the DNA repair enzyme
MRE11, to initiate DNA repair, within 13 seconds.
γH2AX, the phosphorylated form of
H2AX is also involved in the early steps leading to chromatin decondensation after DNA double-strand breaks. The
histone variant H2AX constitutes about 10% of the H2A histones in human chromatin.
γH2AX (H2AX phosphorylated on serine 139) can be detected as soon as
20 seconds after irradiation of cells (with DNA double-strand break
formation), and half maximum accumulation of γH2AX occurs in one minute. The extent of chromatin with phosphorylated γH2AX is about two million base pairs at the site of a DNA double-strand break. γH2AX does not, itself, cause chromatin decondensation, but within 30 seconds of irradiation,
RNF8 protein can be detected in association with γH2AX. RNF8 mediates extensive chromatin decondensation, through its subsequent interaction with
CHD4, a component of the nucleosome remodeling and deacetylase complex
NuRD.
DDB2 occurs in a heterodimeric complex with
DDB1. This complex further complexes with the
ubiquitin ligase protein
CUL4A and with
PARP1.
This larger complex rapidly associates with UV-induced damage within
chromatin, with half-maximum association completed in 40 seconds. The PARP1 protein, attached to both DDB1 and DDB2, then
PARylates (creates a poly-ADP ribose chain) on DDB2 that attracts the DNA remodeling protein
ALC1. Action of ALC1 relaxes the chromatin at the site of UV damage to DNA. This relaxation allows other proteins in the
nucleotide excision repair pathway to enter the chromatin and repair UV-induced
cyclobutane pyrimidine dimer damages.
After rapid
chromatin remodeling,
cell cycle checkpoints are activated to allow DNA repair to occur before the cell cycle progresses. First, two
kinases,
ATM and
ATR
are activated within 5 or 6 minutes after DNA is damaged. This is
followed by phosphorylation of the cell cycle checkpoint protein
Chk1, initiating its function, about 10 minutes after DNA is damaged.
DNA damage checkpoints
After DNA damage,
cell cycle checkpoints
are activated. Checkpoint activation pauses the cell cycle and gives
the cell time to repair the damage before continuing to divide. DNA
damage checkpoints occur at the
G1/
S and
G2/
M boundaries. An intra-
S checkpoint also exists. Checkpoint activation is controlled by two master
kinases,
ATM and
ATR. ATM responds to DNA double-strand breaks and disruptions in chromatin structure, whereas ATR primarily responds to stalled
replication forks. These kinases
phosphorylate downstream targets in a
signal transduction cascade, eventually leading to cell cycle arrest. A class of checkpoint mediator proteins including
BRCA1,
MDC1, and
53BP1 has also been identified. These proteins seem to be required for transmitting the checkpoint activation signal to downstream proteins.
DNA damage checkpoint is a
signal transduction pathway that blocks
cell cycle progression in G1, G2 and
metaphase and slows down the rate of S phase progression when
DNA is damaged. It leads to a pause in cell cycle allowing the cell time to repair the damage before continuing to divide.
Checkpoint Proteins can be separated into four groups:
phosphatidylinositol 3-kinase (PI3K)-like
protein kinase,
proliferating cell nuclear antigen
(PCNA)-like group, two serine/threonine(S/T) kinases and their
adaptors. Central to all DNA damage induced checkpoints responses is a
pair of large protein kinases belonging to the first group of PI3K-like
protein kinases-the ATM (
Ataxia telangiectasia mutated)
and ATR (Ataxia- and Rad-related) kinases, whose sequence and functions
have been well conserved in evolution. All DNA damage response requires
either ATM or ATR because they have the ability to bind to the
chromosomes
at the site of DNA damage, together with accessory proteins that are
platforms on which DNA damage response components and DNA repair
complexes can be assembled.
The prokaryotic SOS response
The
SOS response is the changes in
gene expression in
Escherichia coli and other bacteria in response to extensive DNA damage. The
prokaryotic SOS system is regulated by two key proteins:
LexA and
RecA. The LexA
homodimer is a
transcriptional repressor that binds to
operator sequences commonly referred to as SOS boxes. In
Escherichia coli it is known that LexA regulates transcription of approximately 48 genes including the lexA and recA genes. The SOS response is known to be widespread in the Bacteria domain, but it is mostly absent in some bacterial phyla, like the
Spirochetes.
The most common cellular signals activating the SOS response are regions of single-stranded DNA (ssDNA), arising from stalled
replication forks or double-strand breaks, which are processed by
DNA helicase to separate the two DNA strands. In the initiation step, RecA protein binds to ssDNA in an
ATP hydrolysis driven reaction creating RecA–ssDNA filaments. RecA–ssDNA filaments activate LexA auto
protease
activity, which ultimately leads to cleavage of LexA dimer and
subsequent LexA degradation. The loss of LexA repressor induces
transcription of the SOS genes and allows for further signal induction,
inhibition of cell division and an increase in levels of proteins
responsible for damage processing.
In
Escherichia coli, SOS boxes are 20-nucleotide long sequences near promoters with
palindromic
structure and a high degree of sequence conservation. In other classes
and phyla, the sequence of SOS boxes varies considerably, with different
length and composition, but it is always highly conserved and one of
the strongest short signals in the genome.
The high information content of SOS boxes permits differential binding
of LexA to different promoters and allows for timing of the SOS
response. The lesion repair genes are induced at the beginning of SOS
response. The error-prone translesion polymerases, for example, UmuCD'2
(also called DNA polymerase V), are induced later on as a last resort.
Once the DNA damage is repaired or bypassed using polymerases or
through recombination, the amount of single-stranded DNA in cells is
decreased, lowering the amounts of RecA filaments decreases cleavage
activity of LexA homodimer, which then binds to the SOS boxes near
promoters and restores normal gene expression.
Eukaryotic transcriptional responses to DNA damage
Eukaryotic
cells exposed to DNA damaging agents also activate important defensive
pathways by inducing multiple proteins involved in DNA repair,
cell cycle checkpoint
control, protein trafficking and degradation. Such genome wide
transcriptional response is very complex and tightly regulated, thus
allowing coordinated global response to damage. Exposure of
yeast Saccharomyces cerevisiae to DNA damaging agents results in overlapping but distinct transcriptional profiles. Similarities to environmental
shock response
indicates that a general global stress response pathway exist at the
level of transcriptional activation. In contrast, different human cell
types respond to damage differently indicating an absence of a common
global response. The probable explanation for this difference between
yeast and human cells may be in the
heterogeneity of
mammalian
cells. In an animal different types of cells are distributed among
different organs that have evolved different sensitivities to DNA
damage.
In general global response to DNA damage involves expression of multiple genes responsible for
postreplication repair, homologous recombination, nucleotide excision repair,
DNA damage checkpoint,
global transcriptional activation, genes controlling mRNA decay, and
many others. A large amount of damage to a cell leaves it with an
important decision: undergo apoptosis and die, or survive at the cost of
living with a modified genome. An increase in tolerance to damage can
lead to an increased rate of survival that will allow a greater
accumulation of mutations. Yeast Rev1 and human polymerase η are members
of [Y family translesion DNA
polymerases
present during global response to DNA damage and are responsible for
enhanced mutagenesis during a global response to DNA damage in
eukaryotes.
Aging
Pathological effects of poor DNA repair

DNA repair rate is an important determinant of cell pathology
Experimental animals with genetic deficiencies in DNA repair often show decreased life span and increased cancer incidence. For example, mice deficient in the dominant NHEJ pathway and in telomere maintenance mechanisms get
lymphoma and infections more often, and, as a consequence, have shorter lifespans than wild-type mice.
In similar manner, mice deficient in a key repair and transcription
protein that unwinds DNA helices have premature onset of aging-related
diseases and consequent shortening of lifespan.
However, not every DNA repair deficiency creates exactly the predicted
effects; mice deficient in the NER pathway exhibited shortened life span
without correspondingly higher rates of mutation.
If the rate of DNA damage exceeds the capacity of the cell to
repair it, the accumulation of errors can overwhelm the cell and result
in early senescence, apoptosis, or cancer. Inherited diseases associated
with faulty DNA repair functioning result in premature aging, increased sensitivity to carcinogens, and correspondingly increased cancer risk (see
below). On the other hand, organisms with enhanced DNA repair systems, such as
Deinococcus radiodurans, the most radiation-resistant known organism, exhibit remarkable resistance to the double-strand break-inducing effects of
radioactivity, likely due to enhanced efficiency of DNA repair and especially NHEJ.
Longevity and caloric restriction

Most life span influencing genes affect the rate of DNA damage
A number of individual genes have been identified as influencing
variations in life span within a population of organisms. The effects of
these genes is strongly dependent on the environment, in particular, on
the organism's diet.
Caloric restriction reproducibly results in extended lifespan in a variety of organisms, likely via
nutrient sensing pathways and decreased
metabolic rate. The molecular mechanisms by which such restriction results in lengthened lifespan are as yet unclear; however, the behavior of many genes known to be
involved in DNA repair is altered under conditions of caloric
restriction. Several agents reported to have anti-aging properties have
been shown to attenuate constitutive level of
mTOR signaling, an evidence of reduction of
metabolic activity, and concurrently to reduce constitutive level of
DNA damage induced by endogenously generated reactive oxygen species.
For example, increasing the
gene dosage of the gene SIR-2, which regulates DNA packaging in the nematode worm
Caenorhabditis elegans, can significantly extend lifespan.
The mammalian homolog of SIR-2 is known to induce downstream DNA repair
factors involved in NHEJ, an activity that is especially promoted under
conditions of caloric restriction. Caloric restriction has been closely linked to the rate of base excision repair in the nuclear DNA of rodents, although similar effects have not been observed in mitochondrial DNA.
The
C. elegans gene AGE-1, an upstream effector of DNA
repair pathways, confers dramatically extended life span under
free-feeding conditions but leads to a decrease in reproductive fitness
under conditions of caloric restriction. This observation supports the
pleiotropy theory of the
biological origins of aging,
which suggests that genes conferring a large survival advantage early
in life will be selected for even if they carry a corresponding
disadvantage late in life.
Medicine and DNA repair modulation
Hereditary DNA repair disorders
Defects in the NER mechanism are responsible for several genetic disorders, including:
Mental retardation often accompanies the latter two disorders, suggesting increased vulnerability of developmental neurons.
Other DNA repair disorders include:
All of the above diseases are often called "segmental
progerias" ("
accelerated aging diseases")
because their victims appear elderly and suffer from aging-related
diseases at an abnormally young age, while not manifesting all the
symptoms of old age.
Cancer
Because
of inherent limitations in the DNA repair mechanisms, if humans lived
long enough, they would all eventually develop cancer. There are at least 34
Inherited human DNA repair gene mutations that increase cancer risk. Many of these mutations cause DNA repair to be less effective than normal. In particular,
Hereditary nonpolyposis colorectal cancer (HNPCC) is strongly associated with specific mutations in the DNA mismatch repair pathway.
BRCA1 and
BRCA2, two important genes whose mutations confer a hugely increased risk of breast cancer on carriers, are both associated with a large number of DNA repair pathways, especially NHEJ and homologous recombination.
Cancer therapy procedures such as
chemotherapy and
radiotherapy
work by overwhelming the capacity of the cell to repair DNA damage,
resulting in cell death. Cells that are most rapidly dividing – most
typically cancer cells – are preferentially affected. The side-effect is
that other non-cancerous but rapidly dividing cells such as progenitor
cells in the gut, skin, and hematopoietic system are also affected.
Modern cancer treatments attempt to localize the DNA damage to cells and
tissues only associated with cancer, either by physical means
(concentrating the therapeutic agent in the region of the tumor) or by
biochemical means (exploiting a feature unique to cancer cells in the
body). In the context of therapies targeting DNA damage response genes,
the latter approach has been termed 'synthetic lethality'.
Perhaps the most well-known of these 'synthetic lethality' drugs is the poly(ADP-ribose) polymerase 1 (
PARP1) inhibitor
olaparib,
which was approved by the Food and Drug Administration in 2015 for the
treatment in women of BRCA-defective ovarian cancer. Tumor cells with
partial loss of DNA damage response (specifically,
homologous recombination
repair) are dependent on another mechanism – single-strand break repair
– which is a mechanism consisting, in part, of the PARP1 gene product.
Olaparib
is combined with chemotherapeutics to inhibit single-strand break
repair induced by DNA damage caused by the co-administered chemotherapy.
Tumor cells relying on this residual DNA repair mechanism are unable to
repair the damage and hence are not able to survive and proliferate,
whereas normal cells can repair the damage with the functioning
homologous recombination mechanism.
Many other drugs for use against other residual DNA repair
mechanisms commonly found in cancer are currently under investigation.
However, synthetic lethality therapeutic approaches have been questioned
due to emerging evidence of acquired resistance, achieved through
rewiring of DNA damage response pathways and reversion of
previously-inhibited defects.
DNA repair defects in cancer
It
has become apparent over the past several years that the DNA damage
response acts as a barrier to the malignant transformation of
preneoplastic cells. Previous studies have shown an elevated DNA damage response in cell-culture models with oncogene activation and preneoplastic colon adenomas.
DNA damage response mechanisms trigger cell-cycle arrest, and attempt
to repair DNA lesions or promote cell death/senescence if repair is not
possible. Replication stress is observed in preneoplastic cells due to
increased proliferation signals from oncogenic mutations.
Replication stress
is characterized by: increased replication initiation/origin firing;
increased transcription and collisions of transcription-replication
complexes; nucleotide deficiency; increase in reactive oxygen species
(ROS).
Replication stress, along with the selection for inactivating
mutations in DNA damage response genes in the evolution of the tumor,
leads to downregulation and/or loss of some DNA damage response
mechanisms, and hence loss of DNA repair and/or senescence/programmed
cell death. In experimental mouse models, loss of DNA damage
response-mediated cell senescence was observed after using a
short hairpin RNA (shRNA) to inhibit the double-strand break response kinase ataxia telangiectasia (
ATM), leading to increased tumor size and invasiveness. Humans born with inherited defects in DNA repair mechanisms (for example,
Li-Fraumeni syndrome) have a higher cancer risk.
The prevalence of DNA damage response mutations differs across
cancer types; for example, 30% of breast invasive carcinomas have
mutations in genes involved in homologous recombination.
In cancer, downregulation is observed across all DNA damage response
mechanisms (base excision repair (BER), nucleotide excision repair
(NER), DNA mismatch repair (MMR), homologous recombination repair (HR),
non-homologous end joining (NHEJ) and translesion DNA synthesis (TLS). As well as mutations to DNA damage repair genes, mutations also arise in the genes responsible for arresting the
cell cycle
to allow sufficient time for DNA repair to occur, and some genes are
involved in both DNA damage repair and cell cycle checkpoint control,
for example ATM and checkpoint kinase 2 (CHEK2) – a tumor suppressor
that is often absent or downregulated in non-small cell lung cancer.
|
HR |
NHEJ |
SSA |
FA |
BER |
NER |
MMR
|
|---|
| ATM |
x |
x |
x |
|
|
|
|
| ATR |
x |
x |
x |
|
|
|
|
| PAXIP |
x |
x |
|
|
|
|
|
| RPA |
x |
|
x |
|
|
x |
|
| BRCA1 |
x |
|
|
x |
|
|
|
| BRCA2 |
x |
|
|
x |
|
|
|
| RAD51 |
x |
|
|
x |
|
|
|
| RFC |
x |
|
|
|
x |
x |
|
| XRCC1 |
|
|
|
|
x |
x |
|
| PCNA |
|
|
|
|
x |
x |
x
|
| PARP1 |
|
x |
|
|
x |
|
|
| ERCC1 |
x |
|
x |
x |
|
x |
|
| MSH3 |
x |
|
x |
|
|
|
x
|
Table: Genes involved in DNA damage response pathways and frequently
mutated in cancer (HR = homologous recombination; NHEJ = non-homologous
end joining; SSA = single-strand annealing; FA = fanconi anemia pathway;
BER = base excision repair; NER = nucleotide excision repair; MMR =
mismatch repair)
Epigenetic DNA repair defects in cancer
Classically,
cancer has been viewed as a set of diseases that are driven by
progressive genetic abnormalities that include mutations in
tumour-suppressor genes and oncogenes, and chromosomal aberrations.
However, it has become apparent that cancer is also driven by
epigenetic alterations.
Epigenetic alterations refer to functionally relevant
modifications to the genome that do not involve a change in the
nucleotide sequence. Examples of such modifications are changes in
DNA methylation (hypermethylation and hypomethylation) and
histone modification, changes in chromosomal architecture (caused by inappropriate expression of proteins such as
HMGA2 or
HMGA1) and changes caused by
microRNAs. Each of these epigenetic alterations serves to regulate gene expression without altering the underlying
DNA sequence. These changes usually remain through
cell divisions, last for multiple cell generations, and can be considered to be epimutations (equivalent to mutations).
While large numbers of epigenetic alterations are found in
cancers, the epigenetic alterations in DNA repair genes, causing reduced
expression of DNA repair proteins, appear to be particularly important.
Such alterations are thought to occur early in progression to cancer
and to be a likely cause of the
genetic instability characteristic of cancers.
Reduced expression of DNA repair genes causes deficient DNA
repair. When DNA repair is deficient DNA damages remain in cells at a
higher than usual level and these excess damages cause increased
frequencies of mutation or epimutation. Mutation rates increase
substantially in cells defective in
DNA mismatch repair or in
homologous recombinational repair (HRR). Chromosomal rearrangements and aneuploidy also increase in HRR defective cells.
Higher levels of DNA damage not only cause increased mutation,
but also cause increased epimutation. During repair of DNA double strand
breaks, or repair of other DNA damages, incompletely cleared sites of
repair can cause epigenetic gene silencing.
Deficient expression of DNA repair proteins due to an inherited
mutation can cause increased risk of cancer. Individuals with an
inherited impairment in any of 34 DNA repair genes have an increased risk of cancer, with some defects causing up to a 100% lifetime chance of cancer (e.g. p53 mutations).
However, such germline mutations (which cause highly penetrant cancer
syndromes) are the cause of only about 1 percent of cancers.
Frequencies of epimutations in DNA repair genes
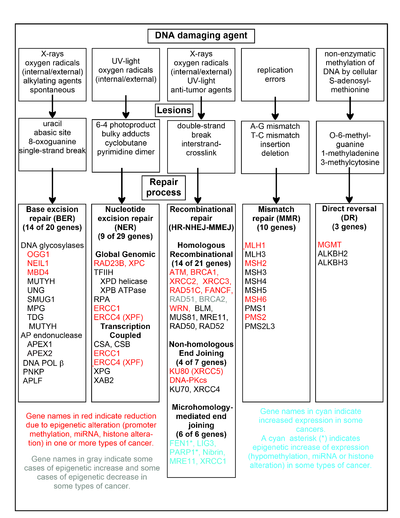
A
chart of common DNA damaging agents, examples of lesions they cause in
DNA, and pathways used to repair these lesions. Also shown are many of
the genes in these pathways, an indication of which genes are
epigenetically regulated to have reduced (or increased) expression in
various cancers. It also shows genes in the error prone
microhomology-mediated end joining pathway with increased expression in
various cancers.
Deficiencies in DNA repair enzymes are occasionally caused by a newly
arising somatic mutation in a DNA repair gene, but are much more
frequently caused by epigenetic alterations that reduce or silence
expression of DNA repair genes. For example, when 113 colorectal cancers
were examined in sequence, only four had a
missense mutation in the DNA repair gene
MGMT, while the majority had reduced MGMT expression due to methylation of the MGMT promoter region (an epigenetic alteration).
Five different studies found that between 40% and 90% of colorectal
cancers have reduced MGMT expression due to methylation of the MGMT
promoter region.
Similarly, out of 119 cases of mismatch repair-deficient colorectal cancers that lacked DNA repair gene
PMS2
expression, PMS2 was deficient in 6 due to mutations in the PMS2 gene,
while in 103 cases PMS2 expression was deficient because its pairing
partner
MLH1 was repressed due to promoter methylation (PMS2 protein is unstable in the absence of MLH1). In the other 10 cases, loss of PMS2 expression was likely due to epigenetic overexpression of the
microRNA,
miR-155, which down-regulates MLH1.
In a further example, epigenetic defects were found in various
cancers (e.g. breast, ovarian, colorectal and head and neck). Two or
three deficiencies in the expression of
ERCC1,
XPF or PMS2 occur simultaneously in the majority of 49 colon cancers evaluated by Facista et al.
The chart in this section shows some frequent DNA damaging
agents, examples of DNA lesions they cause, and the pathways that deal
with these DNA damages. At least 169 enzymes are either directly
employed in DNA repair or influence DNA repair processes. Of these, 83 are directly employed in repairing the 5 types of DNA damages illustrated in the chart.
Some of the more well studied genes central to these repair
processes are shown in the chart. The gene designations shown in red,
gray or cyan indicate genes frequently epigenetically altered in various
types of cancers. Wikipedia articles on each of the genes highlighted
by red, gray or cyan describe the epigenetic alteration(s) and the
cancer(s) in which these epimutations are found. Review articles, and broad experimental survey articles also document most of these epigenetic DNA repair deficiencies in cancers.
Red-highlighted genes are frequently reduced or silenced by
epigenetic mechanisms in various cancers. When these genes have low or
absent expression, DNA damages can accumulate. Replication errors past
these damages can lead to increased mutations and, ultimately, cancer. Epigenetic repression of DNA repair genes in
accurate DNA repair pathways appear to be central to
carcinogenesis.
The two gray-highlighted genes
RAD51 and
BRCA2, are required for
homologous recombinational
repair. They are sometimes epigenetically over-expressed and sometimes
under-expressed in certain cancers. As indicated in the Wikipedia
articles on
RAD51 and
BRCA2,
such cancers ordinarily have epigenetic deficiencies in other DNA
repair genes. These repair deficiencies would likely cause increased
unrepaired DNA damages. The over-expression of
RAD51 and
BRCA2 seen in these cancers may reflect selective pressures for compensatory
RAD51 or
BRCA2
over-expression and increased homologous recombinational repair to at
least partially deal with such excess DNA damages. In those cases where
RAD51 or
BRCA2 are under-expressed, this would itself lead to increased unrepaired DNA damages. Replication errors past these damages could cause increased mutations and cancer, so that under-expression of
RAD51 or
BRCA2 would be carcinogenic in itself.
Cyan-highlighted genes are in the
microhomology-mediated end joining (MMEJ) pathway and are up-regulated in cancer. MMEJ is an additional error-prone
inaccurate
repair pathway for double-strand breaks. In MMEJ repair of a
double-strand break, an homology of 5–25 complementary base pairs
between both paired strands is sufficient to align the strands, but
mismatched ends (flaps) are usually present. MMEJ removes the extra
nucleotides (flaps) where strands are joined, and then ligates the
strands to create an intact DNA double helix. MMEJ almost always
involves at least a small deletion, so that it is a mutagenic pathway.
FEN1,
the flap endonuclease in MMEJ, is epigenetically increased by promoter
hypomethylation and is over-expressed in the majority of cancers of the
breast, prostate, stomach, neuroblastomas, pancreas, and lung. PARP1 is also over-expressed when its promoter region
ETS site is
epigenetically hypomethylated, and this contributes to progression to endometrial cancer and BRCA-mutated serous ovarian cancer. Other genes in the
MMEJ pathway are also over-expressed in a number of cancers (see
MMEJ for summary), and are also shown in cyan.
Genome-wide distribution of DNA repair in human somatic cells
Differential
activity of DNA repair pathways across various regions of the human
genome causes mutations to be very unevenly distributed within tumor
genomes.
In particular, the gene-rich, early-replicating regions of the human
genome exhibit lower mutation frequencies than the gene-poor,
late-replicating
heterochromatin. One mechanism underlying this involves the
histone modification H3K36me3, which can recruit
mismatch repair proteins, thereby lowering mutation rates in
H3K36me3-marked regions. Another important mechanism concerns
nucleotide excision repair, which can be recruited by the transcription machinery, lowering somatic mutation rates in active genes and other open chromatin regions.
Evolution
The basic processes of DNA repair are highly
conserved among both
prokaryotes and
eukaryotes and even among
bacteriophages (
viruses which infect
bacteria); however, more complex organisms with more complex genomes have correspondingly more complex repair mechanisms. The ability of a large number of protein
structural motifs
to catalyze relevant chemical reactions has played a significant role
in the elaboration of repair mechanisms during evolution. For an
extremely detailed review of hypotheses relating to the evolution of DNA
repair, see.
The
fossil record indicates that single-cell life began to proliferate on the planet at some point during the
Precambrian period, although exactly when recognizably modern life first emerged is unclear.
Nucleic acids
became the sole and universal means of encoding genetic information,
requiring DNA repair mechanisms that in their basic form have been
inherited by all extant life forms from their common ancestor. The
emergence of Earth's oxygen-rich atmosphere (known as the "
oxygen catastrophe") due to
photosynthetic organisms, as well as the presence of potentially damaging
free radicals in the cell due to
oxidative phosphorylation, necessitated the evolution of DNA repair mechanisms that act specifically to counter the types of damage induced by
oxidative stress.
Rate of evolutionary change
On
some occasions, DNA damage is not repaired, or is repaired by an
error-prone mechanism that results in a change from the original
sequence. When this occurs,
mutations may propagate into the genomes of the cell's progeny. Should such an event occur in a
germ line cell that will eventually produce a
gamete, the mutation has the potential to be passed on to the organism's offspring. The rate of
evolution
in a particular species (or, in a particular gene) is a function of the
rate of mutation. As a consequence, the rate and accuracy of DNA repair
mechanisms have an influence over the process of evolutionary change.
DNA damage protection and repair does not influence the rate of
adaptation by gene regulation and by recombination and selection of
alleles. On the other hand, DNA damage repair and protection does
influence the rate of accumulation of irreparable, advantageous, code
expanding, inheritable mutations, and slows down the evolutionary
mechanism for expansion of the genome of organisms with new
functionalities. The tension between evolvability and mutation repair
and protection needs further investigation.
Technology
A technology named clustered regularly interspaced short palindromic repeat (shortened to
CRISPR-Cas9)
was discovered in 2012. The new technology allows anyone with
molecular biology training to alter the genes of any species with
precision, by inducing DNA damage at a specific point and then altering
DNA repair mechanisms to insert new genes.
It is cheaper, more efficient, and more precise than other
technologies. With the help of CRISPR–Cas9, parts of a genome can be
edited by scientists by removing, adding, or altering parts in a DNA
sequence.