| Influenza A virus subtype H5N1 | |
|---|---|
| Virus classification | |
| (unranked): | Virus |
| Realm: | Riboviria |
| Phylum: | Negarnaviricota |
| Class: | Insthoviricetes |
| Order: | Articulavirales |
| Family: | Orthomyxoviridae |
| Genus: | Alphainfluenzavirus |
| Species: | Influenza A virus |
| Serotype: | Influenza A virus subtype H5N1 |
| Notable strains | |
 |
Influenza A virus subtype H5N1 (A/H5N1) is a subtype of the influenza A virus which can cause illness in humans and many other animal species. A bird-adapted strain of H5N1, called HPAI A(H5N1) for highly pathogenic avian influenza virus of type A of subtype H5N1, is the highly pathogenic causative agent of H5N1 flu, commonly known as avian influenza ("bird flu"). It is enzootic (maintained in the population) in many bird populations, especially in Southeast Asia. One strain of HPAI A(H5N1) is spreading globally after first appearing in Asia. It is epizootic (an epidemic in nonhumans) and panzootic (affecting animals of many species, especially over a wide area), killing tens of millions of birds and spurring the culling of hundreds of millions of others to stem its spread. Many references to "bird flu" and H5N1 in the popular media refer to this strain.
According to the World Health Organization (WHO) and the United Nations Food and Agriculture Organization, H5N1 pathogenicity is gradually continuing to rise in endemic areas, but the avian influenza disease situation in farmed birds is being held in check by vaccination, and there is "no evidence of sustained human-to-human transmission" of the virus. Eleven outbreaks of H5N1 were reported worldwide in June 2008, in five countries (China, Egypt, Indonesia, Pakistan and Vietnam) compared to 65 outbreaks in June 2006, and 55 in June 2007. The global HPAI situation significantly improved in the first half of 2008, but the FAO reports that imperfect disease surveillance systems mean that occurrence of the virus remains underestimated and underreported. In July 2013, the WHO announced a total of 630 confirmed human cases which resulted in the deaths of 375 people since 2003.
Several H5N1 vaccines have been developed and approved, and stockpiled by a number of countries, including the United States (in its National Stockpile), Britain, France, Canada, and Australia, for use in an emergency.
Research has shown that a highly contagious strain of H5N1, one that might allow airborne transmission between mammals, can be reached in only a few mutations, raising concerns about a pandemic and bioterrorism.
Overview
HPAI
A(H5N1) is considered an avian disease, although there is some evidence
of limited human-to-human transmission of the virus.
A risk factor for contracting the virus is handling of infected
poultry, but transmission of the virus from infected birds to humans has
been characterized as inefficient. Still, around 60% of humans known to have been infected with the Asian strain of HPAI A(H5N1) have died from it, and H5N1 may mutate or reassort into a strain capable of efficient human-to-human transmission. In 2003, world-renowned virologist Robert G. Webster
published an article titled "The world is teetering on the edge of a
pandemic that could kill a large fraction of the human population" in American Scientist. He called for adequate resources to fight what he sees as a major world threat to possibly billions of lives. On September 29, 2005, David Nabarro,
the newly appointed Senior United Nations System Coordinator for Avian
and Human Influenza, warned the world that an outbreak of avian
influenza could kill anywhere between 5 million and 150 million people. Experts have identified key events (creating new clades,
infecting new species, spreading to new areas) marking the progression
of an avian flu virus towards becoming pandemic, and many of those key
events have occurred more rapidly than expected.
Due to the high lethality and virulence of HPAI A(H5N1), its endemic presence, its increasingly large host
reservoir, and its significant ongoing mutations, in 2006, the H5N1
virus has been regarded to be the world's largest pandemic threat, and
billions of dollars are being spent researching H5N1 and preparing for a
potential influenza pandemic. At least 12 companies and 17 governments are developing prepandemic influenza vaccines
in 28 different clinical trials that, if successful, could turn a
deadly pandemic infection into a nondeadly one. Full-scale production of
a vaccine
that could prevent any illness at all from the strain would require at
least three months after the virus's emergence to begin, but it is hoped
that vaccine production could increase until one billion doses were
produced by one year after the initial identification of the virus.
H5N1 may cause more than one influenza pandemic, as it is expected to continue mutating in birds regardless of whether humans develop herd immunity to a future pandemic strain. Influenza pandemics from its genetic offspring may include influenza A virus subtypes other than H5N1.
While genetic analysis of the H5N1 virus shows that influenza pandemics
from its genetic offspring can easily be far more lethal than the Spanish flu pandemic, planning for a future influenza pandemic is based on what can be done and there is no higher Pandemic Severity Index level than a Category 5 pandemic which, roughly speaking, is any pandemic as bad as the Spanish flu or worse; and for which all intervention measures are to be used.
Signs and symptoms
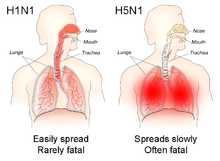
The different sites of infection (shown in red) of seasonal H1N1 versus avian H5N1 influences their lethality and ability to spread.
In general, humans who catch a humanized influenza A virus (a human flu virus of type A) usually have symptoms that include fever, cough, sore throat, muscle aches, conjunctivitis, and, in severe cases, breathing problems and pneumonia that may be fatal. The severity of the infection depends in large part on the state of the infected persons' immune systems
and whether they had been exposed to the strain before (in which case
they would be partially immune). No one knows if these or other symptoms
will be the symptoms of a humanized H5N1 flu.
The avian influenza hemagglutinin binds alpha 2-3 sialic acid receptors, while human influenza hemagglutinins bind alpha 2-6 sialic acid receptors. This means when the H5N1 strain infects humans, it will replicate in the lower respiratory tract, and consequently will cause viral pneumonia. There is as yet no human form of H5N1, so all humans who have caught it so far have caught avian H5N1.
The reported mortality rate of highly pathogenic H5N1 avian influenza in a human is high; WHO
data indicate 60% of cases classified as H5N1 resulted in death.
However, there is some evidence the actual mortality rate of avian flu
could be much lower, as there may be many people with milder symptoms
who do not seek treatment and are not counted.
In one case, a boy with H5N1 experienced diarrhea followed rapidly by a coma without developing respiratory or flu-like symptoms. There have been studies of the levels of cytokines in humans infected by the H5N1 flu virus. Of particular concern is elevated levels of tumor necrosis factor-alpha,
a protein associated with tissue destruction at sites of infection and
increased production of other cytokines. Flu virus-induced increases in
the level of cytokines is also associated with flu symptoms, including
fever, chills, vomiting and headache. Tissue damage associated with
pathogenic flu virus infection can ultimately result in death. The inflammatory cascade triggered by H5N1 has been called a 'cytokine storm' by some, because of what seems to be a positive feedback process of damage to the body resulting from immune system stimulation. H5N1 induces higher levels of cytokines than the more common flu virus types.
In birds
Clinical
signs of H5N1 in birds range from mild—decrease in egg production,
nasal discharge, coughing and sneezing—to severe, including loss of
coordination, energy, and appetite; soft-shelled or misshapen eggs;
purple discoloration of the wattles, head, eyelids, combs, and hocks;
and diarrhea. Sometimes the first noticeable sign is sudden death.
Genetics
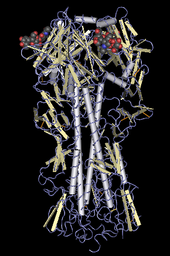
The H in H5N1 stands for "hemagglutinin", as depicted in this molecular model
The first known strain of HPAI A(H5N1) (called A/chicken/Scotland/59)
killed two flocks of chickens in Scotland in 1959, but that strain was
very different from the highly pathogenic strain of H5N1. The dominant
strain of HPAI A(H5N1) in 2004 evolved from 1999 to 2002 creating the Z genotype. It has also been called "Asian lineage HPAI A(H5N1)".
Asian lineage HPAI A(H5N1) is divided into two antigenic clades. "Clade 1 includes human and bird isolates from Vietnam, Thailand, and Cambodia and bird isolates from Laos and Malaysia. Clade 2 viruses were first identified in bird isolates from China, Indonesia, Japan, and South Korea before spreading westward to the Middle East, Europe, and Africa.
The clade 2 viruses have been primarily responsible for human H5N1
infections that have occurred during late 2005 and 2006, according to
WHO. Genetic analysis has identified six subclades of clade 2, three of
which have a distinct geographic distribution and have been implicated
in human infections: Map
- Subclade 1, Indonesia
- Subclade 2, Europe, Middle East, and Africa (called EMA)
- Subclade 3, China"
A 2007 study focused on the EMA subclade has shed further light on
the EMA mutations. "The 36 new isolates reported here greatly expand the
amount of whole-genome sequence data available from recent avian
influenza (H5N1) isolates. Before our project, GenBank contained only 5
other complete genomes from Europe for the 2004–2006 period, and it
contained no whole genomes from the Middle East or northern Africa. Our
analysis showed several new findings. First, all European, Middle
Eastern, and African samples fall into a clade that is distinct from
other contemporary Asian clades, all of which share common ancestry with
the original 1997 Hong Kong strain. Phylogenetic trees built on each of
the 8 segments show a consistent picture of 3 lineages, as illustrated
by the HA tree shown in Figure 1. Two of the clades contain exclusively
Vietnamese isolates; the smaller of these, with 5 isolates, we label V1;
the larger clade, with 9 isolates, is V2. The remaining 22 isolates all
fall into a third, clearly distinct clade, labeled EMA, which comprises
samples from Europe, the Middle East, and Africa. Trees for the other 7
segments display a similar topology, with clades V1, V2, and EMA
clearly separated in each case. Analyses of all available complete
influenza (H5N1) genomes and of 589 HA sequences placed the EMA clade as
distinct from the major clades circulating in People's Republic of
China, Indonesia, and Southeast Asia."
Terminology
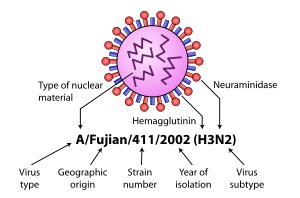
H5N1 isolates are identified like this actual HPAI A(H5N1) example, A/chicken/Nakorn-Patom/Thailand/CU-K2/04(H5N1):
- A stands for the genus of influenza (A, B or C).
- chicken is the animal species the isolate was found in (note: human isolates lack this component term and are thus identified as human isolates by default)
- Nakorn-Patom/Thailand is the place this specific virus was isolated
- CU-K2 is the laboratory reference number that identifies it from other influenza viruses isolated at the same place and year
- 04 represents the year of isolation 2004
- H5 stands for the fifth of several known types of the protein hemagglutinin.
- N1 stands for the first of several known types of the protein neuraminidase.
Other examples include: A/duck/Hong Kong/308/78(H5N3), A/avian/NY/01(H5N2), A/chicken/Mexico/31381-3/94(H5N2), and A/shoveler/Egypt/03(H5N2).
As with other avian flu viruses, H5N1 has strains called "highly
pathogenic" (HP) and "low-pathogenic" (LP). Avian influenza viruses that
cause HPAI are highly virulent,
and mortality rates in infected flocks often approach 100%. LPAI
viruses have negligible virulence, but these viruses can serve as
progenitors to HPAI viruses. The strain of H5N1 responsible for the
deaths of birds across the world is an HPAI strain; all other strains of
H5N1, including a North American strain that causes no disease at all
in any species, are LPAI strains. All HPAI strains identified to date
have involved H5 and H7 subtypes. The distinction concerns pathogenicity
in poultry, not humans. Normally, a highly pathogenic avian virus is
not highly pathogenic to either humans or nonpoultry birds. This deadly
strain of H5N1 is unusual in being deadly to so many species, including
some, like domestic cats, never previously susceptible to any influenza virus.
The N in H5N1 stands for "Neuraminidase", the protein depicted in this ribbon diagram
H5N1 is a subtype of the species Influenza A virus of the genus Alphainfluenzavirus of the family Orthomyxoviridae. Like all other influenza A subtypes, the H5N1 subtype is an RNA virus. It has a segmented genome of eight negative sense, single-strands of RNA, abbreviated as PB2, PB1, PA, HA, NP, NA, MP and NS.
HA codes for hemagglutinin, an antigenic glycoprotein
found on the surface of the influenza viruses and is responsible for
binding the virus to the cell that is being infected. NA codes for neuraminidase, an antigenic glycosylated enzyme found on the surface of the influenza viruses. It facilitates the release of progeny viruses from infected cells.
The hemagglutinin (HA) and neuraminidase (NA) RNA strands specify the
structure of proteins that are most medically relevant as targets for
antiviral drugs and antibodies. HA and NA are also used as the basis for the naming of the different subtypes of influenza A viruses. This is where the H and N come from in H5N1.
Influenza A viruses are significant for their potential for
disease and death in humans and other animals. Influenza A virus
subtypes that have been confirmed in humans, in order of the number of
known human pandemic deaths that they have caused, include:
- H1N1, which caused the 1918 flu pandemic ("Spanish flu") and the 2009 flu pandemic ("swine flu") and is causing seasonal human flu
- H2N2, which caused "Asian flu"
- H3N2, which caused "Hong Kong flu" and causes seasonal human flu
- H5N1, ("bird flu"), which is noted for having a strain (Asian-lineage HPAI H5N1) that kills over half the humans it infects, infecting and killing species that were never known to suffer from influenza viruses before (e.g. cats), being unable to be stopped by culling all involved poultry—some think due to being endemic in wild birds, and causing billions of dollars to be spent in flu pandemic preparation and preventiveness
- H7N7, which has unusual zoonotic potential and killed one person
- H1N2, which is endemic in humans and pigs and causes seasonal human flu
- H9N2, which has infected three people
- H7N2, which has infected two people
- H7N3, which has infected two people
- H10N7, which has infected two people
- H7N9, which as of Feb 2014 has infected 309 people, and lead to 70 deaths
Low pathogenic H5N1
Low
pathogenic avian influenza H5N1 (LPAI H5N1) also called "North
American" H5N1 commonly occurs in wild birds. In most cases, it causes
minor sickness or no noticeable signs of disease in birds. It is not
known to affect humans at all. The only concern about it is that it is
possible for it to be transmitted to poultry and in poultry mutate into a
highly pathogenic strain.
- 1966 – LPAI H5N1 A/Turkey/Ontario/6613/1966(H5N1) was detected in a flock of infected turkeys in Ontario, Canada
- 1975 – LPAI H5N1 was detected in a wild mallard duck and a wild blue goose in Wisconsin.
- 1981 and 1985 – LPAI H5N1 was detected in ducks by the University of Minnesota conducting a sampling procedure in which sentinel ducks were monitored in cages placed in the wild for a short period of time.
- 1983 – LPAI H5N1 was detected in ring-billed gulls in Pennsylvania.
- 1986 – LPAI H5N1 was detected in a wild mallard duck in Ohio.
- 2005 – LPAI H5N1 was detected in ducks in Manitoba, Canada.
- 2008 – LPAI H5N1 was detected in ducks in New Zealand.
- 2009 – LPAI H5N1 was detected in commercial poultry in British Columbia.
"In the past, there was no requirement for reporting or tracking LPAI
H5 or H7 detections in wild birds so states and universities tested
wild bird samples independently of USDA. Because of this, the above list
of previous detections might not be all inclusive of past LPAI H5N1
detections. However, the World Organization for Animal Health (OIE)
recently changed its requirement of reporting detections of avian
influenza. Effective in 2006, all confirmed LPAI H5 and H7 AI subtypes
must be reported to the OIE because of their potential to mutate into
highly pathogenic strains. Therefore, USDA now tracks these detections
in wild birds, backyard flocks, commercial flocks and live bird
markets."
High mutation rate
Influenza viruses have a relatively high mutation rate that is characteristic of RNA viruses. The segmentation of its genome facilitates genetic recombination by segment reassortment in hosts infected with two different strains of influenza viruses at the same time. A previously uncontagious strain may then be able to pass between humans, one of several possible paths to a pandemic.
The ability of various influenza strains to show species-selectivity is largely due to variation in the hemagglutinin genes. Genetic mutations in the hemagglutinin gene that cause single amino acid substitutions can significantly alter the ability of viral hemagglutinin proteins to bind to receptors
on the surface of host cells. Such mutations in avian H5N1 viruses can
change virus strains from being inefficient at infecting human cells to
being as efficient in causing human infections as more common human
influenza virus types.
This doesn't mean that one amino acid substitution can cause a
pandemic, but it does mean that one amino acid substitution can cause an
avian flu virus that is not pathogenic in humans to become pathogenic
in humans.
Influenza A virus subtype H3N2
is endemic in pigs in China, and has been detected in pigs in Vietnam,
increasing fears of the emergence of new variant strains. The dominant
strain of annual flu virus in January 2006 was H3N2, which is now resistant to the standard antiviral drugs amantadine and rimantadine.
The possibility of H5N1 and H3N2 exchanging genes through reassortment
is a major concern. If a reassortment in H5N1 occurs, it might remain an
H5N1 subtype, or it could shift subtypes, as H2N2 did when it evolved into the Hong Kong Flu strain of H3N2.
Both the H2N2 and H3N2 pandemic strains contained avian influenza
virus RNA segments. "While the pandemic human influenza viruses of 1957
(H2N2) and 1968 (H3N2) clearly arose through reassortment between human
and avian viruses, the influenza virus causing the 'Spanish flu' in
1918 appears to be entirely derived from an avian source".
Prevention
Vaccine
There are several H5N1 vaccines
for several of the avian H5N1 varieties, but the continual mutation of
H5N1 renders them of limited use to date: while vaccines can sometimes
provide cross-protection against related flu strains, the best
protection would be from a vaccine specifically produced for any future
pandemic flu virus strain. Daniel R. Lucey, co-director of the Biohazardous Threats and Emerging Diseases graduate program at Georgetown University has made this point, "There is no H5N1 pandemic so there can be no pandemic vaccine".
However, "pre-pandemic vaccines" have been created; are being refined
and tested; and do have some promise both in furthering research and
preparedness for the next pandemic.
Vaccine manufacturing companies are being encouraged to increase
capacity so that if a pandemic vaccine is needed, facilities will be
available for rapid production of large amounts of a vaccine specific to
a new pandemic strain.
Public health
"The United States is collaborating closely with eight international organizations, including the World Health Organization (WHO), the Food and Agriculture Organization of the United Nations (FAO), the World Organization for Animal Health
(OIE), and 88 foreign governments to address the situation through
planning, greater monitoring, and full transparency in reporting and
investigating avian influenza occurrences. The United States and these
international partners have led global efforts to encourage countries to
heighten surveillance for outbreaks in poultry and significant numbers
of deaths in migratory birds and to rapidly introduce containment
measures. The U.S. Agency for International Development (USAID) and the U.S. Department of State, the U.S. Department of Health and Human Services (HHS), and Agriculture
(USDA) are coordinating future international response measures on
behalf of the White House with departments and agencies across the
federal government".
Together steps are being taken to "minimize the risk of further
spread in animal populations", "reduce the risk of human infections",
and "further support pandemic planning and preparedness".
Ongoing detailed mutually coordinated onsite surveillance and
analysis of human and animal H5N1 avian flu outbreaks are being
conducted and reported by the USGS National Wildlife Health Center, the Centers for Disease Control and Prevention, the World Health Organization, the European Commission, and others.
Treatment
There is no highly effective treatment for H5N1 flu, but oseltamivir (commercially marketed by Roche
as Tamiflu), can sometimes inhibit the influenza virus from spreading
inside the user's body. This drug has become a focus for some
governments and organizations trying to prepare for a possible H5N1
pandemic. On April 20, 2006, Roche AG announced that a stockpile of three million treatment courses of Tamiflu are waiting at the disposal of the World Health Organization to be used in case of a flu pandemic; separately Roche donated two million courses to the WHO for use in developing nations that may be affected by such a pandemic but lack the ability to purchase large quantities of the drug.
However, WHO expert Hassan al-Bushra has said:
- "Even now, we remain unsure about Tamiflu's real effectiveness. As for a vaccine, work cannot start on it until the emergence of a new virus, and we predict it would take six to nine months to develop it. For the moment, we cannot by any means count on a potential vaccine to prevent the spread of a contagious influenza virus, whose various precedents in the past 90 years have been highly pathogenic".
Animal and lab studies suggest that Relenza (zanamivir),
which is in the same class of drugs as Tamiflu, may also be effective
against H5N1. In a study performed on mice in 2000, "zanamivir was shown
to be efficacious in treating avian influenza viruses H9N2, H6N1, and H5N1 transmissible to mammals".
In addition, mice studies suggest the combination of zanamivir,
celecoxib and mesalazine looks promising producing a 50% survival rate
compared to no survival in the placebo arm.
While no one knows if zanamivir will be useful or not on a yet to exist
pandemic strain of H5N1, it might be useful to stockpile zanamivir as
well as oseltamivir in the event of an H5N1 influenza pandemic. Neither
oseltamivir nor zanamivir can be manufactured in quantities that would
be meaningful once efficient human transmission starts.
In September, 2006, a WHO scientist announced that studies had
confirmed cases of H5N1 strains resistant to Tamiflu and Amantadine. Tamiflu-resistant strains have also appeared in the EU, which remain sensitive to Relenza.
Epidemiology
The earliest infections of humans by H5N1 coincided with an epizootic (an epidemic in nonhumans) of H5N1 influenza in Hong Kong's poultry population in 1997. This panzootic
(a disease affecting animals of many species, especially over a wide
area) outbreak was stopped by the killing of the entire domestic poultry
population within the territory. However, the disease has continued to
spread; outbreaks were reported in Asia again in 2003. On December 21,
2009 the WHO announced a total of 447 cases which resulted in the deaths
of 263.
Contagiousness
Highly pathogenic H5N1
Countries with humans, poultry and wild birds killed by H5N1
Countries with poultry or wild birds killed by H5N1 and has reported human cases of H5N1
Countries with poultry or wild birds killed by H5N1
H5N1 is easily transmissible between birds, facilitating a potential global spread of H5N1.
While H5N1 undergoes mutation and reassortment, creating variations
which can infect species not previously known to carry the virus, not
all of these variant forms can infect humans. H5N1 as an avian virus
preferentially binds to a type of galactose
receptors that populate the avian respiratory tract from the nose to
the lungs and are virtually absent in humans, occurring only in and
around the alveoli,
structures deep in the lungs where oxygen is passed to the blood.
Therefore, the virus is not easily expelled by coughing and sneezing,
the usual route of transmission.
H5N1 is mainly spread by domestic poultry,
both through the movements of infected birds and poultry products and
through the use of infected poultry manure as fertilizer or feed. Humans
with H5N1 have typically caught it from chickens, which were in turn
infected by other poultry or waterfowl. Migrating waterfowl (wild ducks, geese and swans) carry H5N1, often without becoming sick.
Many species of birds and mammals can be infected with HPAI A(H5N1),
but the role of animals other than poultry and waterfowl as
disease-spreading hosts is unknown.
According to a report by the World Health Organization,
H5N1 may be spread indirectly. The report stated the virus may
sometimes stick to surfaces or get kicked up in fertilizer dust to
infect people.
Virulence
H5N1 has mutated into a variety of strains
with differing pathogenic profiles, some pathogenic to one species but
not others, some pathogenic to multiple species. Each specific known
genetic variation is traceable to a virus isolate of a specific case of
infection. Through antigenic drift,
H5N1 has mutated into dozens of highly pathogenic varieties divided
into genetic clades which are known from specific isolates, but all
belong to genotype Z of avian influenza virus H5N1, now the dominant
genotype. H5N1 isolates found in Hong Kong
in 1997 and 2001 were not consistently transmitted efficiently among
birds and did not cause significant disease in these animals. In 2002,
new isolates of H5N1 were appearing within the bird population of Hong
Kong. These new isolates caused acute disease, including severe
neurological dysfunction and death in ducks. This was the first reported case of lethal influenza virus infection in wild aquatic birds since 1961.
Genotype Z emerged in 2002 through reassortment from earlier highly pathogenic genotypes of H5N1 that first infected birds in China in 1996, and first infected humans in Hong Kong in 1997.
Genotype Z is endemic in birds in Southeast Asia, has created at least
two clades that can infect humans, and is spreading across the globe in
bird populations. Mutations occurring within this genotype are
increasing their pathogenicity.
Birds are also able to shed the virus for longer periods of time before
their death, increasing the transmissibility of the virus.
Transmission and host range
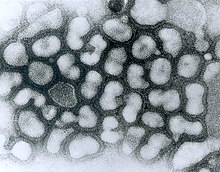
Transmission electron micrograph (TEM) of negatively stained Influenza A virus particles (small and white) attached to host cells (large and irregular) (late passage). (Source: Dr. Erskine Palmer, Centers for Disease Control and Prevention Public Health Image Library)
Infected birds transmit H5N1 through their saliva, nasal secretions, feces and blood.
Other animals may become infected with the virus through direct contact
with these bodily fluids or through contact with surfaces contaminated
with them. H5N1 remains infectious after over 30 days at 0 °C (32 °F)
(over one month at freezing temperature) or 6 days at 37 °C (99 °F) (one
week at human body temperature); at ordinary temperatures it lasts in
the environment for weeks. In Arctic temperatures, it does not degrade
at all.
Because migratory birds are among the carriers of the highly
pathogenic H5N1 virus, it is spreading to all parts of the world. H5N1
is different from all previously known highly pathogenic avian flu
viruses in its ability to be spread by animals other than poultry.
In October 2004, researchers discovered H5N1 is far more dangerous than was previously believed. Waterfowl were revealed to be directly spreading this highly pathogenic strain to chickens, crows, pigeons,
and other birds, and the virus was increasing its ability to infect
mammals, as well. From this point on, avian flu experts increasingly
referred to containment as a strategy that can delay, but not ultimately
prevent, a future avian flu pandemic.
"Since 1997, studies of influenza A (H5N1) indicate that these
viruses continue to evolve, with changes in antigenicity and internal
gene constellations; an expanded host range in avian species and the
ability to infect felids; enhanced pathogenicity in experimentally
infected mice and ferrets, in which they cause systemic infections; and
increased environmental stability."
The New York Times, in an article on transmission of H5N1
through smuggled birds, reports Wade Hagemeijer of Wetlands
International stating, "We believe it is spread by both bird migration
and trade, but that trade, particularly illegal trade, is more
important".
On September 27, 2007, researchers reported the H5N1 bird flu
virus can also pass through a pregnant woman's placenta to infect the
fetus. They also found evidence of what doctors had long suspected—the
virus not only affects the lungs, but also passes throughout the body
into the gastrointestinal tract, the brain, liver, and blood cells.
In May 2013, North Korea confirmed a H5N1 bird flu outbreak that forced authorities to kill over 160,000 ducks in Pyongyang.
H5N1 transmission studies in ferrets (2011)
Novel, contagious strains of H5N1 were created by Ron Fouchier of the Erasmus Medical Center
in Rotterdam, the Netherlands, who first presented his work to the
public at an influenza conference in Malta in September 2011. Three
mutations were introduced into the H5N1 virus genome, and the virus was
then passed from the noses of infected ferrets to the noses of uninfected ones, which was repeated 10 times.
After these 10 passages the H5N1 virus had acquired the ability of
transmission between ferrets via aerosols or respiratory droplets.
After Fouchier offered an article describing this work to the leading academic journal Science, the US National Science Advisory Board for Biosecurity
(NSABB) recommended against publication of the full details of the
study, and the one submitted to Nature by Yoshihiro Kawaoka of the
University of Wisconsin describing related work. However, after
additional consultations at the World Health Organization and by the
NSABB, the NSABB reversed its position and recommended publication of
revised versions of the two papers. However, then the Dutch government
declared that this type of manuscripts required Fouchier to apply for
an export permit in the light of EU directive 428/2009 on dual use
goods.
After much controversy surrounding the publishing of his research,
Fouchier complied (under formal protest) with Dutch government demands
to obtain a special permit for submitting his manuscript, and his research appeared in a special issue of the journal Science devoted to H5N1.
The papers by Fouchier and Kawaoka conclude that it is entirely
possible that a natural chain of mutations could lead to an H5N1 virus
acquiring the capability of airborne transmission between mammals, and
that a H5N1 influenza pandemic would not be impossible.
In May 2013, it was reported that scientists at the Harbin Veterinary Research Institute in Harbin, China had created H5N1 strains which passed between guinea pigs.
Society and culture
H5N1 has had a significant effect on human society, especially the financial, political, social, and personal responses to both actual and predicted deaths in birds, humans, and other animals. Billions of U.S. dollars are being raised and spent to research H5N1 and prepare for a potential avian influenza pandemic. Over $10 billion have been spent and over 200 million birds have been killed to try to contain H5N1.
People have reacted by buying less chicken, causing poultry sales and prices to fall.
Many individuals have stockpiled supplies for a possible flu pandemic.
International health officials and other experts have pointed out that
many unknown questions still hover around the disease.
Dr. David Nabarro,
Chief Avian Flu Coordinator for the United Nations, and former Chief of
Crisis Response for the World Health Organization has described himself
as "quite scared" about H5N1's potential impact on humans. Nabarro has
been accused of being alarmist before, and on his first day in his role
for the United Nations, he proclaimed the avian flu could kill 150
million people. In an interview with the International Herald Tribune, Nabarro compares avian flu to AIDS in Africa, warning that underestimations led to inappropriate focus for research and intervention.