The thermoelectric effect is the direct conversion of temperature differences to electric voltage and vice versa via a thermocouple.[1]
A thermoelectric device creates voltage when there is a different
temperature on each side. Conversely, when a voltage is applied to it,
it creates a temperature difference. At the atomic scale, an applied
temperature gradient causes charge carriers in the material to diffuse from the hot side to the cold side.
This effect can be used to generate electricity, measure temperature or change the temperature of objects. Because the direction of heating and cooling is determined by the polarity of the applied voltage, thermoelectric devices can be used as temperature controllers.
The term "thermoelectric effect" encompasses three separately identified effects: the Seebeck effect, Peltier effect, and Thomson effect. The Seebeck and Peltier effects are different manifestations of the same physical process; textbooks may refer to this process as the Peltier–Seebeck effect (the separation derives from the independent discoveries by French physicist Jean Charles Athanase Peltier and Baltic German physicist Thomas Johann Seebeck). The Thomson effect is an extension of the Peltier–Seebeck model and is credited to Lord Kelvin.
Joule heating, the heat that is generated whenever a current is passed through a resistive material, is related, though it is not generally termed a thermoelectric effect. The Peltier–Seebeck and Thomson effects are thermodynamically reversible,[2] whereas Joule heating is not.
This effect can be used to generate electricity, measure temperature or change the temperature of objects. Because the direction of heating and cooling is determined by the polarity of the applied voltage, thermoelectric devices can be used as temperature controllers.
The term "thermoelectric effect" encompasses three separately identified effects: the Seebeck effect, Peltier effect, and Thomson effect. The Seebeck and Peltier effects are different manifestations of the same physical process; textbooks may refer to this process as the Peltier–Seebeck effect (the separation derives from the independent discoveries by French physicist Jean Charles Athanase Peltier and Baltic German physicist Thomas Johann Seebeck). The Thomson effect is an extension of the Peltier–Seebeck model and is credited to Lord Kelvin.
Joule heating, the heat that is generated whenever a current is passed through a resistive material, is related, though it is not generally termed a thermoelectric effect. The Peltier–Seebeck and Thomson effects are thermodynamically reversible,[2] whereas Joule heating is not.
Seebeck effect
A thermoelectric circuit composed of materials of different Seebeck coefficients (p-doped and n-doped semiconductors), configured as a thermoelectric generator. If the load resistor at the bottom is replaced with a voltmeter, the circuit then functions as a temperature-sensing thermocouple.
The Seebeck effect is the conversion of heat directly into electricity at the junction of different types of wire. Originally discovered in 1794 by Italian scientist Alessandro Volta,[3][note 1] it is named after the Baltic German physicist Thomas Johann Seebeck, who in 1821 independently rediscovered it.[4] It was observed that a compass needle would be deflected by a closed loop formed by two different metals joined in two places, with a temperature difference between the joints. This was because the electron energy levels in each metal shifted differently and a potential difference between the junctions created an electrical current and therefore a magnetic field around the wires. Seebeck did not recognize that there was an electric current involved, so he called the phenomenon "thermomagnetic effect". Danish physicist Hans Christian Ørsted rectified the oversight and coined the term "thermoelectricity".[5]
The Seebeck effect is a classic example of an electromotive force (emf) and leads to measurable currents or voltages in the same way as any other emf. Electromotive forces modify Ohm's law by generating currents even in the absence of voltage differences (or vice versa); the local current density is given by
 is the local voltage,[6] and
is the local voltage,[6] and  is the local conductivity. In general, the Seebeck effect is described locally by the creation of an electromotive field
is the local conductivity. In general, the Seebeck effect is described locally by the creation of an electromotive field is the Seebeck coefficient (also known as thermopower), a property of the local material, and
is the Seebeck coefficient (also known as thermopower), a property of the local material, and  is the temperature gradient.
is the temperature gradient.The Seebeck coefficients generally vary as function of temperature and depend strongly on the composition of the conductor. For ordinary materials at room temperature, the Seebeck coefficient may range in value from −100 μV/K to +1,000 μV/K.
If the system reaches a steady state, where
 , then the voltage gradient is given simply by the emf:
, then the voltage gradient is given simply by the emf:  . This simple relationship, which does not depend on conductivity, is used in the thermocouple
to measure a temperature difference; an absolute temperature may be
found by performing the voltage measurement at a known reference
temperature. A metal of unknown composition can be classified by its
thermoelectric effect if a metallic probe of known composition is kept
at a constant temperature and held in contact with the unknown sample
that is locally heated to the probe temperature. It is used commercially
to identify metal alloys. Thermocouples in series form a thermopile. Thermoelectric generators are used for creating power from heat differentials.
. This simple relationship, which does not depend on conductivity, is used in the thermocouple
to measure a temperature difference; an absolute temperature may be
found by performing the voltage measurement at a known reference
temperature. A metal of unknown composition can be classified by its
thermoelectric effect if a metallic probe of known composition is kept
at a constant temperature and held in contact with the unknown sample
that is locally heated to the probe temperature. It is used commercially
to identify metal alloys. Thermocouples in series form a thermopile. Thermoelectric generators are used for creating power from heat differentials.Peltier effect
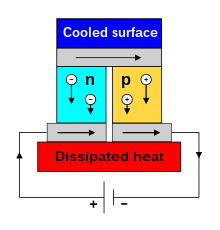
The Seebeck circuit configured as a thermoelectric cooler
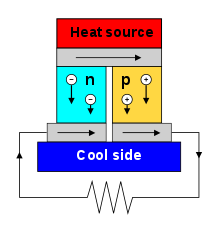
The Peltier effect is the presence of heating or cooling at an electrified junction of two different conductors and is named after French physicist Jean Charles Athanase Peltier, who discovered it in 1834.[7] When a current is made to flow through a junction between two conductors, A and B, heat may be generated or removed at the junction. The Peltier heat generated at the junction per unit time is
 and
and  are the Peltier coefficients of conductors A and B, and
are the Peltier coefficients of conductors A and B, and  is the electric current (from A to B). The total heat generated is not
determined by the Peltier effect alone, as it may also be influenced by
Joule heating and thermal-gradient effects (see below).
is the electric current (from A to B). The total heat generated is not
determined by the Peltier effect alone, as it may also be influenced by
Joule heating and thermal-gradient effects (see below).The Peltier coefficients represent how much heat is carried per unit charge. Since charge current must be continuous across a junction, the associated heat flow will develop a discontinuity if
 and
and  are different. The Peltier effect can be considered as the back-action counterpart to the Seebeck effect (analogous to the back-emf
in magnetic induction): if a simple thermoelectric circuit is closed,
then the Seebeck effect will drive a current, which in turn (by the
Peltier effect) will always transfer heat from the hot to the cold
junction. The close relationship between Peltier and Seebeck effects can
be seen in the direct connection between their coefficients:
are different. The Peltier effect can be considered as the back-action counterpart to the Seebeck effect (analogous to the back-emf
in magnetic induction): if a simple thermoelectric circuit is closed,
then the Seebeck effect will drive a current, which in turn (by the
Peltier effect) will always transfer heat from the hot to the cold
junction. The close relationship between Peltier and Seebeck effects can
be seen in the direct connection between their coefficients:  (see below).
(see below).A typical Peltier heat pump involves multiple junctions in series, through which a current is driven. Some of the junctions lose heat due to the Peltier effect, while others gain heat. Thermoelectric heat pumps exploit this phenomenon, as do thermoelectric cooling devices found in refrigerators.
Thomson effect
In different materials, the Seebeck coefficient is not constant in temperature, and so a spatial gradient in temperature can result in a gradient in the Seebeck coefficient. If a current is driven through this gradient, then a continuous version of the Peltier effect will occur. This Thomson effect was predicted and subsequently observed in 1851 by Lord Kelvin (William Thomson).[8] It describes the heating or cooling of a current-carrying conductor with a temperature gradient.If a current density
 is passed through a homogeneous conductor, the Thomson effect predicts a heat production rate per unit volume
is passed through a homogeneous conductor, the Thomson effect predicts a heat production rate per unit volume is the temperature gradient, and
is the temperature gradient, and  is the Thomson coefficient. The Thomson coefficient is related to the Seebeck coefficient as
is the Thomson coefficient. The Thomson coefficient is related to the Seebeck coefficient as  (see below). This equation, however, neglects Joule heating and ordinary thermal conductivity (see full equations below).
(see below). This equation, however, neglects Joule heating and ordinary thermal conductivity (see full equations below).Full thermoelectric equations
Often, more than one of the above effects is involved in the operation of a real thermoelectric device. The Seebeck effect, Peltier effect, and Thomson effect can be gathered together in a consistent and rigorous way, described here; the effects of Joule heating and ordinary heat conduction are included as well. As stated above, the Seebeck effect generates an electromotive force, leading to the current equation[9] , is[9]
, is[9] is the thermal conductivity. The first term is the Fourier's heat conduction law, and the second term shows the energy carried by currents. The third term,
is the thermal conductivity. The first term is the Fourier's heat conduction law, and the second term shows the energy carried by currents. The third term,  , is the heat added from an external source (if applicable).
, is the heat added from an external source (if applicable).In the case where the material has reached a steady state, the charge and temperature distributions are stable, so one must have both
 and
and  . Using these facts and the second Thomson relation (see below), the heat equation then can be simplified to
. Using these facts and the second Thomson relation (see below), the heat equation then can be simplified to at junction) and Thomson (
at junction) and Thomson ( in thermal gradient) effects. Combined with the Seebeck equation for
in thermal gradient) effects. Combined with the Seebeck equation for  , this can be used to solve for the steady-state voltage and temperature profiles in a complicated system.
, this can be used to solve for the steady-state voltage and temperature profiles in a complicated system.If the material is not in a steady state, a complete description will also need to include dynamic effects such as relating to electrical capacitance, inductance, and heat capacity.
Thomson relations
In 1854, Lord Kelvin found relationships between the three coefficients, implying that the Thomson, Peltier, and Seebeck effects are different manifestations of one effect (uniquely characterized by the Seebeck coefficient).[10]The first Thomson relation is[9]
 is the absolute temperature,
is the absolute temperature,  is the Thomson coefficient,
is the Thomson coefficient,  is the Peltier coefficient, and
is the Peltier coefficient, and  is the Seebeck coefficient. This relationship is easily shown given
that the Thomson effect is a continuous version of the Peltier effect.
Using the second relation (described next), the first Thomson relation
becomes
is the Seebeck coefficient. This relationship is easily shown given
that the Thomson effect is a continuous version of the Peltier effect.
Using the second relation (described next), the first Thomson relation
becomes  .
.The second Thomson relation is
The Thomson coefficient is unique among the three main thermoelectric coefficients because it is the only one directly measurable for individual materials. The Peltier and Seebeck coefficients can only be easily determined for pairs of materials; hence, it is difficult to find values of absolute Seebeck or Peltier coefficients for an individual material.
If the Thomson coefficient of a material is measured over a wide temperature range, it can be integrated using the Thomson relations to determine the absolute values for the Peltier and Seebeck coefficients. This needs to be done only for one material, since the other values can be determined by measuring pairwise Seebeck coefficients in thermocouples containing the reference material and then adding back the absolute Seebeck coefficient of the reference material.