From Wikipedia, the free encyclopedia
B vitamins are a class of water-soluble vitamins that play important roles in cell metabolism. Though these vitamins share similar names, research shows that they are chemically distinct vitamins that often coexist in the same foods. In general, supplements containing all eight are referred to as a vitamin B complex. Individual B vitamin supplements are referred to by the specific name of each vitamin (e.g., B1, B2, B3 etc.).
List of B vitamins
- Vitamin B1 (thiamine)
- Vitamin B2 (riboflavin)
- Vitamin B3 (niacin or nicotinic acid)
- Vitamin B5 (pantothenic acid)
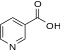
- Vitamin B6 (pyridoxine, pyridoxal, pyridoxamine)
- Vitamin B7 (biotin)
- Vitamin B9 (folic acid)
- Vitamin B12 (various cobalamins; commonly cyanocobalamin or methylcobalamin in vitamin supplements)
B vitamin molecular functions
| Vitamin | Name | Structure | Molecular Function |
|---|---|---|---|
| Vitamin B1 | Thiamine |  |
Thiamine plays a central role in the generation of energy from carbohydrates. It is involved in RNA and DNA production, as well as nerve function. Its active form is a coenzyme called thiamine pyrophosphate (TPP), which takes part in the conversion of pyruvate to acetyl coenzyme A (CoA) in metabolism.[1] |
| Vitamin B2 | Riboflavin |  |
Riboflavin is involved in the energy production for the electron transport chain, the citric acid cycle, as well as the catabolism of fatty acids (beta oxidation)[2] |
| Vitamin B3 | Niacin | Niacin is composed of two structures: nicotinic acid and nicotinamide. There are two co-enzyme forms of niacin: nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP). Both play an important role in energy transfer reactions in the metabolism of glucose, fat and alcohol.[3]
NAD carries hydrogens and their electrons during metabolic reactions, including the pathway from the citric acid cycle to the electron transport chain. NADP is a coenzyme in lipid and nucleic acid synthesis.[4] |
|
| Vitamin B5 | Pantothenic acid | Pantothenic acid is involved in the oxidation of fatty acids and carbohydrates. Coenzyme A, which can be synthesised from pantothenic acid, is involved in the synthesis of amino acids, fatty acids, ketones, cholesterol,[5] phospholipids, steroid hormones, neurotransmitters (such as acetylcholine), and antibodies.[6] | |
| Vitamin B6 | pyridoxine, pyridoxal, pyridoxamine |  |
The active form pyridoxal 5'-phosphate (PLP) (depicted) serves as a cofactor in many enzyme reactions mainly in amino acid metabolism including biosynthesis of neurotransmitters. |
| Vitamin B7 | Biotin |  |
Biotin plays a key role in the metabolism of lipids, proteins and carbohydrates. It is a critical co-enzyme of four carboxylases: acetyl CoA carboxylase, which is involved in the synthesis of fatty acids from acetate; propionyl CoA carboxylase, involved in gluconeogenesis; β-methylcrotonyl CoA carboxylase, involved in the metabolism of leucin; and pyruvate CoA carboxylase, which is involved in the metabolism of energy, amino acids and cholesterol.[7] |
| Vitamin B9 | Folic acid | Folic acid acts as a co-enzyme in the form of tetrahydrofolate (THF), which is involved in the transfer of single-carbon units in the metabolism of nucleic acids and amino acids. THF is involved in pyrimidine nucleotide synthesis, so is needed for normal cell division, especially during pregnancy and infancy, which are times of rapid growth. Folate also aids in erythropoiesis, the production of red blood cells.[8] | |
| Vitamin B12 | Cobalamin | 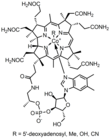 |
Vitamin B12 is involved in the cellular metabolism of carbohydrates, proteins and lipids. It is essential in the production of blood cells in bone marrow, and for nerve sheaths and proteins.[9] Vitamin B12 functions as a co-enzyme in intermediary metabolism for the methionine synthase reaction with methylcobalamin, and the methylmalonyl CoA mutase reaction with adenosylcobalamin.[10] |
B vitamin deficiency
Several named vitamin deficiency diseases may result from the lack of sufficient B vitamins. Deficiencies of other B vitamins result in symptoms that are not part of a named deficiency disease.| Vitamin | Name | Deficiency effects |
| Vitamin B1 | thiamine | Deficiency causes beriberi. Symptoms of this disease of the nervous system include weight loss, emotional disturbances, Wernicke's encephalopathy (impaired sensory perception), weakness and pain in the limbs, periods of irregular heartbeat, and edema (swelling of bodily tissues). Heart failure and death may occur in advanced cases. Chronic thiamin deficiency can also cause Korsakoff's syndrome, an irreversible dementia characterized by amnesia and compensatory confabulation. |
| Vitamin B2 | riboflavin | Deficiency causes ariboflavinosis. Symptoms may include cheilosis (cracks in the lips), high sensitivity to sunlight, angular cheilitis, glossitis (inflammation of the tongue), seborrheic dermatitis or pseudo-syphilis (particularly affecting the scrotum or labia majora and the mouth), pharyngitis (sore throat), hyperemia, and edema of the pharyngeal and oral mucosa. |
| Vitamin B3 | niacin | Deficiency, along with a deficiency of tryptophan causes pellagra. Symptoms include aggression, dermatitis, insomnia, weakness, mental confusion, and diarrhea. In advanced cases, pellagra may lead to dementia and death (the 3(+1) Ds: dermatitis, diarrhea, dementia, and death). |
| Vitamin B5 | pantothenic acid | Deficiency can result in acne and paresthesia, although it is uncommon. |
| Vitamin B6 | pyridoxine, pyridoxal, pyridoxamine |
Main article: vitamin B6 § Deficiency
|
| Vitamin B7 | biotin | Deficiency does not typically cause symptoms in adults but may lead to impaired growth and neurological disorders in infants. Multiple carboxylase deficiency, an inborn error of metabolism, can lead to biotin deficiency even when dietary biotin intake is normal. |
| Vitamin B9 | folic acid | Deficiency results in a macrocytic anemia, and elevated levels of homocysteine. Deficiency in pregnant women can lead to birth defects. Supplementation is often recommended during pregnancy. Researchers have shown that folic acid might also slow the insidious effects of age on the brain. |
| Vitamin B12 | cobalamin | Deficiency results in a macrocytic anemia, elevated homocysteine, peripheral neuropathy, memory loss and other cognitive deficits. It is most likely to occur among elderly people, as absorption through the gut declines with age; the autoimmune disease pernicious anemia is another common cause. It can also cause symptoms of mania and psychosis. In rare extreme cases, paralysis can result. |
B vitamin side effects
Because water-soluble B vitamins are eliminated in the urine, taking large doses of certain B vitamins usually only produces transient side-effects. General side effects may include restlessness, nausea and insomnia. These side-effects are almost always caused by dietary supplements and not foodstuffs.
| Vitamin | Name | Tolerable Upper Intake Level | Harmful effects |
| Vitamin B1 | thiamine | None[11] | No known toxicity from oral intake. There are some reports of anaphylaxis caused by high-dose thiamin injections into the vein or muscle. However, the doses were greater than the quantity humans can physically absorb from oral intake.[11] |
| Vitamin B2 | riboflavin | None.[12] | No evidence of toxicity based on limited human and animal studies. The only evidence of adverse effects associated with riboflavin comes from in vitro studies showing the production of reactive oxygen species (free radicals) when riboflavin was exposed to intense visible and UV light.[12] |
| Vitamin B3 | niacin | 35 mg/day from supplements, drugs or fortified food[13] | Intake of 3000 mg/day of nicotinamide and 1500 mg/day of nicotinic acid are associated with nausea, vomiting, and signs and symptoms of liver toxicity. Other effects may include glucose intolerance, and (reversible) ocular effects. Additionally, the nicotinic acid form may cause vasodilatory effects, also known as flushing, including redness of the skin, often accompanied by an itching, tingling, or mild burning sensation, which is also often accompanied by pruritus, headaches, and increased intracranial blood flow, and occasionally accompanied by pain.[13] Medical practitioners prescribe recommended doses up to 2000 mg per day of niacin, usually in time release format, to combat arterial plaque development in cases of high lipid levels.[14] |
| Vitamin B5 | pantothenic acid | None | No known toxicity |
| Vitamin B6 | pyridoxine, pyridoxal, pyridoxamine |
Main article: vitamin B6 § Toxicity
|
Main article: vitamin B6 § Toxicity
|
| Vitamin B7 | biotin | None | No known toxicity |
| Vitamin B9 | folic acid | 1 mg/day [15] | Masks B12 deficiency, which can lead to permanent neurological damage[15] |
| Vitamin B12 | cyanocobalamin | None established.[16] | Acne-like rash [causality is not conclusively established].[16][17] |
B vitamin sources
B vitamins are found in whole unprocessed foods. Processed carbohydrates such as sugar and white flour tend to have lower B vitamin than their unprocessed counterparts. For this reason, it is required by law in many countries (including the United States) that the B vitamins thiamine, riboflavin, niacin, and folic acid be added back to white flour after processing. This is sometimes called "Enriched Flour" on food labels. B vitamins are particularly concentrated in meat such as turkey, tuna and liver.[18] Good sources for B vitamins include legumes (pulses or beans), whole grains, potatoes, bananas, chili peppers, tempeh, nutritional yeast, brewer's yeast, and molasses. Although the yeast used to make beer results in beers being a source of B vitamins,[19] their bioavailability ranges from poor to negative as drinking ethanol inhibits absorption of thiamine (B1),[20][21] riboflavin (B2),[22] niacin (B3),[23] biotin (B7),[24] and folic acid (B9).[25][26] In addition, each of the preceding studies further emphasizes that elevated consumption of beer and other ethanol-based drinks results in a net deficit of those B vitamins and the health risks associated with such deficiencies.The B12 vitamin is of note because it is not available from plant products, making B12 deficiency a legitimate concern for vegans. Manufacturers of plant-based foods will sometimes report B12 content, leading to confusion about what sources yield B12. The confusion arises because the standard US Pharmacopeia (USP) method for measuring the B12 content does not measure the B12 directly. Instead, it measures a bacterial response to the food. Chemical variants of the B12 vitamin found in plant sources are active for bacteria, but cannot be used by the human body. This same phenomenon can cause significant over-reporting of B12 content in other types of foods as well.[27]
Another popular means of increasing one's vitamin B intake is through the use of dietary supplements. B vitamins are also commonly added to energy drinks, many of which have been marketed with large amounts of B vitamins[28] with claims that this will cause the consumer to "sail through your day without feeling jittery or tense."[28] Some nutritionists have been critical of these claims, pointing out for instance that while B vitamins do "help unlock the energy in foods," most Americans acquire the necessary amounts easily in their diets.[28]
Because they are soluble in water, excess B vitamins (such as may be ingested via supplements) are generally readily excreted, although individual absorption, use and metabolism may vary…"[28] The elderly and athletes may need to supplement their intake of B12 and other B vitamins due to problems in absorption and increased needs for energy production. In cases of severe deficiency B vitamins, especially B12, may also be delivered by injection to reverse deficiencies.[29] Both type 1 and type 2 diabetics may also be advised to supplement thiamine based on high prevalence of low plasma thiamine concentration and increased thiamine clearance associated with diabetes.[30] Also, Vitamin B9 (folic acid) deficiency in early embryo development has been linked to neural tube defects. Thus, women planning to become pregnant are usually encouraged to increase daily dietary folic acid intake and/or take a supplement.[31]
Related nutrients
Many of the following substances have been referred to as vitamins as they were once believed to be vitamins. They are no longer considered as such, and the numbers that were assigned to them now form the "gaps" in the true series of B-complex vitamins described above (e.g., there is no vitamin B4). Some of them, though not essential to humans, are essential in the diets of other organisms; others have no known nutritional value and may even be toxic under certain conditions.- Vitamin B4: can refer to the distinct chemicals choline, adenine, or carnitine.[32][33] Choline is synthesized by the human body, but not sufficiently to maintain good health, and is now considered an essential dietary nutrient.[34] Adenine is a nucleobase synthesized by the human body.[35] Carnitine is an essential dietary nutrient for certain worms, but not for humans.[36]
- Vitamin B8: adenosine monophosphate (AMP), also known as adenylic acid.[37] Vitamin B8 may also refer to inositol.[38]
- Vitamin B10: para-aminobenzoic acid (pABA or PABA), a chemical component of the folate molecule produced by plants and bacteria, and found in many foods.[39][40] It is best known as a UV-blocking sunscreen applied to the skin, and is sometimes taken orally for certain medical conditions.[39][41]
- Vitamin B11: pteryl-hepta-glutamic acid—chick growth factor, which is a form of folic acid. Later found to be one of five folates necessary for humans. Vitamin Bc-conjugate was also found to be PHGA.
- Vitamin B13: orotic acid.[42]
- Vitamin B14: cell proliferant, anti-anemia, rat growth factor, and antitumor pterin phosphate named by Earl R. Norris. Isolated from human urine at 0.33ppm (later in blood), but later abandoned by him as further evidence did not confirm this. He also claimed this was not xanthopterin.
- Vitamin B15: pangamic acid,[42] also known as pangamate. Promoted in various forms as a dietary supplement and drug; considered unsafe and subject to seizure by the US Food and Drug Administration.[43]
- Vitamin B16: dimethylglycine (DMG)[44] is synthesized by the human body in the Citric acid (or Kreb) cycle.
- Vitamin B17: nitrilosides, amygdalin or Laetrile. These substances are found in a number of seeds, sprouts, beans, tubers, and grains. While toxic in large quantities, proponents claim that it is effective in cancer treatment and prevention despite a lack of scientific evidence.[45]
- Vitamin B20: L-carnitine.[44]
- Vitamin Bf: carnitine.[37]
- Vitamin Bm: myo-inositol, also called “mouse antialopaecia factor”.[46]
- Vitamin Bp: “antiperosis factor”, which prevents perosis, a leg disorder, in chicks; can be replaced by choline and manganese salts.[36][37][47]
- Vitamin BT: carnitine.[48][36]
- Vitamin Bv: a type of B6 other than pyridoxine.
- Vitamin BW: a type of biotin other than d-biotin.
- Vitamin Bx: an alternative name for both pABA (see vitamin B10) and pantothenic acid.[36][41]