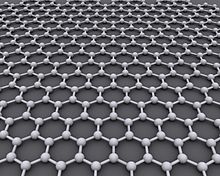
Graphene is an atomic-scale hexagonal lattice made of carbon atoms.
Graphene is a semi-metal with a small overlap between the valence and the conduction bands (zero bandgap material).[1] It is an allotrope (form) of carbon consisting of a single layer of carbon atoms arranged in a hexagonal lattice.[2][3] It is the basic structural element of many other allotropes of carbon, such as graphite, diamond, charcoal, carbon nanotubes and fullerenes.
It can be considered as an indefinitely large aromatic molecule, the ultimate case of the family of flat polycyclic aromatic hydrocarbons.[4]
Graphene has many uncommon properties. It is the strongest material ever tested,[5] efficiently conducts heat and electricity, and is nearly transparent. Graphene shows a large and nonlinear diamagnetism,[6] greater than that of graphite, and can be levitated by neodymium magnets.
Scientists theorized about graphene for years. It had been unintentionally produced in small quantities for centuries, through the use of pencils and other similar graphite applications. It was originally observed in electron microscopes in 1962, but it was studied only while supported on metal surfaces.[7] The material was later rediscovered, isolated, and characterized in 2004 by Andre Geim and Konstantin Novoselov at the University of Manchester.[8][9] Research was informed by existing theoretical descriptions of its composition, structure, and properties.[10] This work resulted in the two winning the Nobel Prize in Physics in 2010 "for groundbreaking experiments regarding the two-dimensional material graphene."[11]
Definition
"Graphene" is a combination of "graphite" and the suffix -ene, named by Hanns-Peter Boehm and colleagues,[12] who produced and observed single-layer carbon foils in 1962.[7]Boehm et al. introduced the term graphene in 1986 to describe single sheets of graphite. Graphene can be considered an "infinite alternant" (only six-member carbon ring) polycyclic aromatic hydrocarbon.[13]
The International Union of Pure and Applied Chemistry (IUPAC) notes: "previously, descriptions such as graphite layers, carbon layers, or carbon sheets have been used for the term graphene...it is incorrect to use for a single layer a term which includes the term graphite, which would imply a three-dimensional structure. The term graphene should be used only when the reactions, structural relations or other properties of individual layers are discussed."[4]
Geim defined "isolated or free-standing graphene" as "graphene is a single atomic plane of graphite, which – and this is essential – is sufficiently isolated from its environment to be considered free-standing."[14] This definition is narrower than the IUPAC definition and refers to cloven, transferred and suspended graphene.[citation needed] Other forms such as graphene grown on various metals, can become free-standing if, for example, suspended or transferred [15][16] to silicon dioxide (SiO
2) or silicon carbide.[17]
Structure
Scanning probe microscopy image of graphene
Graphene is a crystalline allotrope of carbon with 2-dimensional properties. Its carbon atoms are densely packed in a regular atomic-scale chicken wire (hexagonal) pattern.[18]
Each atom has four bonds: one σ bond with each of its three neighbors and one π-bond that is oriented out of plane. The atoms are about 1.42 Å apart.[18]
Graphene's hexagonal lattice can be regarded as two interleaving triangular lattices. This perspective was successfully used to calculate the band structure for a single graphite layer using a tight-binding approximation.[18]
Graphene's stability is due to its tightly packed carbon atoms and a sp2 orbital hybridization – a combination of orbitals s, px and py that constitute the σ-bond. The final pz electron makes up the π-bond. The π-bonds hybridize together to form the π-band and π∗-bands. These bands are responsible for most of graphene's notable electronic properties, via the half-filled band that permits free-moving electrons.[18]
Graphene sheets in solid form usually show evidence in diffraction for graphite's (002) layering. This is true of some single-walled nanostructures.[19] However, unlayered graphene with only (hk0) rings has been found in the core of presolar graphite onions.[20] TEM studies show faceting at defects in flat graphene sheets[21] and suggest a role for two-dimensional crystallization from a melt.
Graphene can self-repair holes in its sheets, when exposed to molecules containing carbon, such as hydrocarbons. Bombarded with pure carbon atoms, the atoms perfectly align into hexagons, completely filling the holes.[22][23]
The atomic structure of isolated, single-layer graphene was studied by TEM on sheets of graphene suspended between bars of a metallic grid.[24] Electron diffraction patterns showed the expected honeycomb lattice. Suspended graphene showed "rippling" of the flat sheet, with amplitude of about one nanometer. These ripples may be intrinsic to the material as a result of the instability of two-dimensional crystals,[25][26][27] or may originate from the ubiquitous dirt seen in all TEM images of graphene. Atomic resolution real-space images of isolated, single-layer graphene on SiO
2 substrates are available[28] via scanning tunneling microscopy (STM). Photoresist residue, which must be removed to obtain atomic-resolution images, may be the "adsorbates" observed in TEM images, and may explain the observed rippling. Rippling on SiO
2 is caused by conformation of graphene to the underlying SiO
2 and is not intrinsic.[28]
Stability
Ab initio calculations show that a graphene sheet is thermodynamically unstable if its size is less than about 20 nm ("graphene is the least stable structure until about 6000 atoms") and becomes the most stable fullerene (as within graphite) only for molecules larger than 24,000 atoms.[29]Analogs
Analogs[30] (also referred to as "artificial graphene") are two-dimensional systems that exhibit similar properties to graphene. Analogs can be systems in which the physics is easier to observe and to manipulate. In those systems, electrons are not always the chosen particles—they might be optical photons,[31] microwave photons,[32] plasmons,[33] microcavity polaritons[34] or even atoms.[35] Also, the honeycomb structure in which those particles evolve can be of a different nature than carbon atoms in graphene. It can be, respectively, a photonic crystal, an array of metallic rods, metallic nanoparticles, a lattice of coupled microcavities or an optical lattice.Properties
Graphene has a theoretical specific surface area (SSA) of 2630 m2/g. This is much larger than that reported to date for carbon black (typically smaller than 900 m2/g) or for carbon nanotubes (CNTs), from ≈100 to 1000 m2/g and is similar to activated carbon.[36]Electronic
GNR band structure for zig-zag orientation. Tightbinding calculations show that zig-zag orientation is always metallic.

GNR band structure
for armchair orientation. Tightbinding calculations show that armchair
orientation can be semiconducting or metallic depending on width
(chirality).
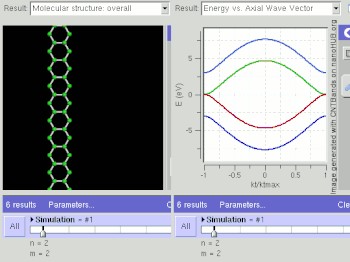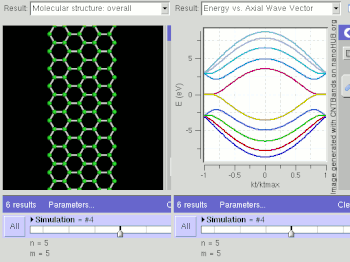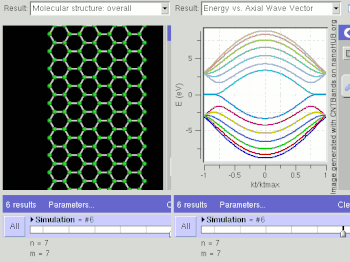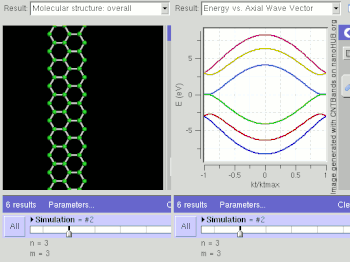
Graphene is a zero-gap semiconductor, because its conduction and valence bands meet at the Dirac points, which are six locations in momentum space, on the edge of the Brillouin zone, divided into two non-equivalent sets of three points. The two sets are labeled K and K'. The sets give graphene a valley degeneracy of gv = 2. By contrast, for traditional semiconductors the primary point of interest is generally Γ, where momentum is zero.[18] However, if the in-plane direction is confined, in which case it is referred to as a nanoribbon, its electronic structure is different. If it is "zig-zag", the bandgap is zero. If it is "armchair", the bandgap is non-zero (see figure).
Graphene displays remarkable electron mobility at room temperature, with reported values in excess of 15000 cm2⋅V−1⋅s−1.[25] Hole and electron mobilities were expected to be nearly identical.[37] The mobility is nearly independent of temperature between 10 K and 100 K,[38][39][40] which implies that the dominant scattering mechanism is defect scattering. Scattering by graphene's acoustic phonons intrinsically limits room temperature mobility to 200000 cm2⋅V−1⋅s−1 at a carrier density of 1012 cm−2,[40][41] 4.5×103 times greater than copper.[42][43]
The corresponding resistivity of graphene sheets would be 10−6 Ω⋅cm. This is less than the resistivity of silver, the lowest otherwise known at room temperature.[44] However, on SiO
2 substrates, scattering of electrons by optical phonons of the substrate is a larger effect than scattering by graphene’s own phonons. This limits mobility to 40000 cm2⋅V−1⋅s−1.[40] Charge transport is affected by adsorption of contaminants such as water and oxygen molecules. This leads to non-repetitive and large hysteresis I-V characteristics.
Electrons propagating through graphene's honeycomb lattice effectively lose their mass, producing quasi-particles that are described by a 2D analogue of the Dirac equation rather than the Schrödinger equation for spin-1⁄2 particles.[45][37]
Superconductivity has been observed in twisted bilayer graphene.
Optical
Graphene's unique optical properties produce an unexpectedly high opacity for an atomic monolayer in vacuum, absorbing πα ≈ 2.3% of red light, where α is the fine-structure constant.[48] This is a consequence of the "unusual low-energy electronic structure of monolayer graphene that features electron and hole conical bands meeting each other at the Dirac point... [which] is qualitatively different from more common quadratic massive bands."[47] Based on the Slonczewski–Weiss–McClure (SWMcC) band model of graphite, the interatomic distance, hopping value and frequency cancel when optical conductance is calculated using Fresnel equations in the thin-film limit.
Although confirmed experimentally, the measurement is not precise enough to improve on other techniques for determining the fine-structure constant.[49]
Multi-parametric surface plasmon resonance was used to characterize both thickness and refractive index of chemical-vapor-deposition (CVD)-grown graphene films. The measured refractive index and extinction coefficient values at 670 nm wavelength are 3.135 and 0.897, respectively. The thickness was determined as 3.7 Å from a 0.5 mm area, which agrees with 3.35 Å reported for layer-to-layer carbon atom distance of graphite crystals.[50]
The method can be used for real-time label-free interactions of graphene with organic and inorganic substances. The existence of unidirectional surface plasmons in the nonreciprocal graphene-based gyrotropic interfaces has been demonstrated theoretically. By efficiently controlling the chemical potential of graphene, the unidirectional working frequency can be continuously tunable from THz to near-infrared and even visible.[51] Particularly, the unidirectional frequency bandwidth can be 1–2 orders of magnitude larger than that in metal under the same magnetic field, which arises from the superiority of extremely small effective electron mass in graphene.
Graphene's band gap can be tuned from 0 to 0.25 eV (about 5 micrometre wavelength) by applying voltage to a dual-gate bilayer graphene field-effect transistor (FET) at room temperature.[52] The optical response of graphene nanoribbons is tunable into the terahertz regime by an applied magnetic field.[53] Graphene/graphene oxide systems exhibit electrochromic behavior, allowing tuning of both linear and ultrafast optical properties.[54]
A graphene-based Bragg grating (one-dimensional photonic crystal) demonstrated its capability for excitation of surface electromagnetic waves in the periodic structure using a 633 nm He–Ne laser as the light source.[55]
Saturable absorption
Such unique absorption could become saturated when the input optical intensity is above a threshold value. This nonlinear optical behavior is termed saturable absorption and the threshold value is called the saturation fluence. Graphene can be saturated readily under strong excitation over the visible to near-infrared region, due to the universal optical absorption and zero band gap. This has relevance for the mode locking of fiber lasers, where fullband mode locking has been achieved by a graphene-based saturable absorber. Due to this special property, graphene has wide application in ultrafast photonics. The optical response of graphene/graphene oxide layers can be tuned electrically.[54][56] Saturable absorption in graphene could occur at the Microwave and Terahertz bands, owing to its wideband optical absorption property. The microwave saturable absorption in graphene demonstrates the possibility of graphene microwave and terahertz photonics devices, such as a microwave saturable absorber, modulator, polarizer, microwave signal processing and broad-band wireless access networks.[57]Nonlinear Kerr effect
Under more intensive laser illumination, graphene could possess a nonlinear phase shift due to the optical nonlinear Kerr effect. Based on a typical open and close aperture z-scan measurement, graphene possesses a nonlinear Kerr coefficient of 10−7 cm2⋅W−1, almost nine orders of magnitude larger than that of bulk dielectrics.[58] This suggests that graphene may be a powerful nonlinear Kerr medium, with the possibility of observing a variety of nonlinear effects, the most important of which is the soliton.[59] A 2016 study has shown that the nonlinear refractive index of graphene is negative.[60] Thermal lens spectroscopy can be used for measuring the thermo-optic coefficient of graphene and inspecting its thermal nonlinearity.[61]Quantum dots
Several techniques can prepare nanostructured graphene, e.g., graphene quantum dots (GQDs); these techniques mainly include electron beam lithography, chemical synthesis, electrochemical preparation, graphene oxide (GO) reduction, C60 catalytic transformation, the microwave assisted hydrothermal method (MAH),[62][63] the Soft-Template method,[64] the hydrothermal method,[65][66][67] and the ultrasonic exfoliation method.[68]Thermal
Thermal conductivity
Thermal transport in graphene is an active area of research which has attracted attention because of the potential for thermal management applications. Early measurements of the thermal conductivity of suspended graphene reported an exceptionally large thermal conductivity of approximately 5300 W⋅m−1⋅K−1,[69] compared with the thermal conductivity of pyrolytic graphite of approximately 2000 W⋅m−1⋅K−1 at room temperature.[70] However, later studies have questioned whether this ultrahigh value was overestimated, and instead measured thermal conductivities between 1500–2500 W⋅m−1⋅K−1 for suspended single layer graphene.[71][72][73][74][75] The large range can be attributed to large measurement uncertainties as well as variations in the graphene quality and processing conditions. In addition, when single-layer graphene is supported on an amorphous material, the thermal conductivity is reduced to about 500–600 W⋅m−1⋅K−1 at room temperature as a result of scattering of graphene lattice waves by the substrate,[76][77] and can be even lower for few layer graphene encased in amorphous oxide.[78] Likewise, polymeric residue can contribute to a similar decrease for suspended graphene to approximately 500–600 W⋅m−1⋅K−1for bilayer graphene.[79]It has been suggested that the isotopic composition, the ratio of 12C to 13C, has a significant impact on thermal conductivity. For example, isotopically pure 12C graphene has higher thermal conductivity than either a 50:50 isotope ratio or the naturally occurring 99:1 ratio.[80] It can be shown by using the Wiedemann–Franz law, that the thermal conduction is phonon-dominated.[69] However, for a gated graphene strip, an applied gate bias causing a Fermi energy shift much larger than kBT can cause the electronic contribution to increase and dominate over the phonon contribution at low temperatures. The ballistic thermal conductance of graphene is isotropic.[81][82]
Potential for this high conductivity can be seen by considering graphite, a 3D version of graphene that has basal plane thermal conductivity of over 1000 W⋅m−1⋅K−1 (comparable to diamond). In graphite, the c-axis (out of plane) thermal conductivity is over a factor of ≈100 smaller due to the weak binding forces between basal planes as well as the larger lattice spacing.[83] In addition, the ballistic thermal conductance of graphene gives the lower limit of the ballistic thermal conductances, per unit circumference and length of carbon nanotubes.[84]
Despite its 2-D nature, graphene has 3 acoustic phonon modes. The two in-plane modes (LA, TA) have a linear dispersion relation, while the out of plane mode (ZA) has a quadratic dispersion relation. Due to this, the T2 dependent thermal conductivity contribution of the linear modes is dominated at low temperatures by the T1.5 contribution of the out-of-plane mode.[84] Some graphene phonon bands display negative Grüneisen parameters (GPs).[85] At low temperatures (where most optical modes with positive GPs are still not excited) the contribution from the negative GPs will be dominant and thermal expansion coefficient (which is directly proportional to GPs) negative. The lowest negative GPs correspond to the lowest transverse acoustic ZA modes. Phonon frequencies for such modes increase with the in-plane lattice parameter since atoms in the layer upon stretching will be less free to move in the z direction. This is similar to the behavior of a stretched string that has vibrations of smaller amplitude and higher frequency. This phenomenon, named "membrane effect", was predicted by Lifshitz in 1952.[86]
Melting point
A prediction that was published in 2015 suggested a melting point of ≈4125 K.[87] Recent[when?] and more sophisticated modelling has increased this temperature to at least 5000 K. At 6000 K (the sun's surface having an effective temperature of 5,777 K)[88] graphene melts into an agglomeration of loosely coupled doubled bonded chains, before becoming a gas.[89]Mechanical
The carbon–carbon bond length in graphene is about 0.142 nanometers.[90] Graphene sheets stack to form graphite with an interplanar spacing of 0.335 nm.[citation needed]Graphene is the strongest material ever tested,[91] with an intrinsic tensile strength of 130.5 GPa and a Young's modulus of 1 TPa (150000000 psi).[91] The Nobel announcement gave the strength as 42 N/m; the mass of 1 m2 as 0.77 mg, and the electrical resistance of a square as 31 Ω.[92]
Large-angle-bent graphene monolayer has been achieved with negligible strain, showing mechanical robustness of the two-dimensional carbon nanostructure. Even with extreme deformation, excellent carrier mobility in monolayer graphene can be preserved.[93]
The spring constant of suspended graphene sheets has been measured using an atomic force microscope (AFM).[91][94] Graphene sheets were suspended over SiO
2 cavities where an atomic force microscope (AFM) tip was used to apply a stress to the sheet to test its mechanical properties. Its spring constant was in the range 1–5 N/m and the stiffness was 0.5 TPa, which differs from that of bulk graphite. These intrinsic properties could lead to applications such as NEMS as pressure sensors and resonators.[95] Due to its large surface energy and out of plane ductility, flat graphene sheets are unstable with respect to scrolling, i.e. bending into a cylindrical shape, which is its lower-energy state.[96]
As is true of all materials, regions of graphene are subject to thermal and quantum fluctuations in relative displacement. Although the amplitude of these fluctuations is bounded in 3D structures (even in the limit of infinite size), the Mermin–Wagner theorem shows that the amplitude of long-wavelength fluctuations grows logarithmically with the scale of a 2D structure and would therefore be unbounded in structures of infinite size.[97][98] Local deformation and elastic strain are negligibly affected by this long-range divergence in relative displacement. It is believed that a sufficiently large 2D structure, in the absence of applied lateral tension, will bend and crumple to form a fluctuating 3D structure. Ripples have been observed in suspended layers of graphene.[24] It has been proposed that the ripples are caused by thermal fluctuations in the material. As a consequence of these dynamical deformations, it is debatable whether graphene is truly a 2D structure.[25][26][27][99] In 2014 it was shown that these ripples, if amplified through the introduction of vacancy defects, can impart a negative Poisson's ratio into graphene, resulting in the thinnest auxetic material known.[100]
Graphene nanosheets can be incorporated into a nickel matrix through a plating process to form Ni-graphene composites on a target substrate. The enhancement in mechanical properties of the composites is attributed to the high interaction between Ni and graphene and the prevention of the dislocation sliding in the Ni matrix by the graphene.[101]
Fracture toughness
Despite its strength, graphene is also relatively brittle, with a fracture toughness of about 4 MPa√m.[102] This indicates that imperfect graphene is likely to crack in a brittle manner like ceramic materials, as opposed to many metallic materials that have fracture toughnesses in the range of 15–50 MPa√m. Graphene shows a greater ability to distribute force from an impact than any known material, ten times that of steel per unit weight.[103] The force[clarification needed] was transmitted at 22.2 kilometres per second (13.8 mi/s).[104]Biological
In 2011 graphene was shown to accelerate the osteogenic differentiation of human mesenchymal stem cells without the use of biochemical inducers.[105]In 2015 graphene was used to create biosensors with epitaxial graphene on silicon carbide. The sensors bind to 8-hydroxydeoxyguanosine (8-OHdG) and is capable of selective binding with antibodies. The presence of 8-OHdG in blood, urine and saliva is commonly associated with DNA damage. Elevated levels of 8-OHdG have been linked to increased risk of several cancers.[106] A commercial version of a graphene biosensor has been used as a protein binding sensor platform.[107]
In 2016 uncoated graphene was shown to serve as a neuro-interface electrode without altering or damaging properties such as signal strength or formation of scar tissue. Graphene electrodes in the body stay significantly more stable than electrodes of tungsten or silicon because of properties such as flexibility, bio-compatibility and conductivity.[108]
Forms
A production unit produces continuous monolayer sheets of high-strength monolayer graphene (HSMG).[109] The process is based on graphene growth on a liquid metal matrix.[110] Bilayer graphene displays the anomalous quantum Hall effect, a tunable band gap[111] and potential for excitonic condensation.[112] Bilayer graphene typically can be found either in twisted configurations where the two layers are rotated relative to each other or graphitic Bernal stacked configurations where half the atoms in one layer lie atop half the atoms in the other.[113] Stacking order and orientation govern its optical and electronic properties. One synthesis method is chemical vapor deposition, which can produce large bilayer regions that almost exclusively conform to a Bernal stack geometry.[113] Superconductivity has been observed in twisted bilayer graphene.Graphene nanoribbons ("nanostripes" in the "zig-zag" orientation), at low temperatures, show spin-polarized metallic edge currents, which suggest spintronics applications. (In the "armchair" orientation, the edges behave like semiconductors.[45])
In 2013, a three-dimensional honeycomb of hexagonally arranged carbon was termed 3D graphene. Self-supporting 3D graphene was produced that year.[114] The discovered nanostructure is a multilayer system of parallel hollow nanochannels located along the surface that displayed quadrangular cross-section. Three dimensional bilayer graphene was reported in 2012[115] and 2014.[116] In 2017, freestanding graphene gyroids with 35 nm and 60 nm unit cells were fabricated via controlled direct chemical vapor deposition. They represent the smallest free standing periodic graphene 3D structures yet produced with a pore size of tens of nm.[117] A graphene gyroid has five percent of the density of steel, yet is ten times as strong with an enormous surface area to volume ratio.[118][119] An aerogel made of graphene layers separated by carbon nanotubes was measured at 0.16 milligrams per cubic centimeter. The material has superior elasticity and absorption: it can recover completely after more than 90% compression, and absorb up to 900 times its weight in oil, at a rate of 68.8 grams per second.[120]
Production
Multiple production techniques have been developed. Isolated 2D crystals cannot be grown via chemical synthesis beyond small sizes even in principle, because the rapid growth of phonon density with increasing lateral size forces 2D crystallites to bend into the third dimension.[14] In all cases, graphene must bond to a substrate to retain its two-dimensional shape.[14]As of 2014, exfoliation produced graphene with the lowest number of defects and highest electron mobility.[43] Geim and Novoselov initially used adhesive tape to pull graphene sheets away from graphite. Achieving single layers typically requires multiple exfoliation steps. After exfoliation the flakes are deposited on a silicon wafer. Alternatively a sharp single-crystal diamond wedge cleave layers from a graphite source.[121] Another method is reduction of graphite oxide monolayer films, e.g. by hydrazine with annealing in argon/hydrogen with an almost intact carbon framework that allows efficient removal of functional groups. Measured charge carrier mobility exceeded 1,000 centimetres (393.70 in)/Vs.[122] Defect-free, unoxidized graphene-containing liquids can be made from graphite using mixers that produce local shear rates greater than 10×104.[123][124] Burning a graphite oxide coated DVD produced a conductive graphene film (1738 siemens per meter) and specific surface area (1520 square meters per gram) that was highly resistant and malleable.[125]
Dispersing graphite in a liquid medium can produce graphene by sonication followed by centrifugation.[126] The addition of dispersants (e.g., graphene quantum dots) can facilitate the exfoliation process, forming aqueous graphene dispersion with high yield.[127] Such process is straightforward, low-cost, and environmentally friendly. Sonicating graphite at the interface of two immiscible liquids, most notably heptane and water, produced macro-scale graphene films.[128] Graphite particles can be corroded in molten salts to form a variety of carbon nanostructures including graphene.[129] Electrochemical synthesis can exfoliate graphene. Varying a pulsed voltage controls thickness, flake area, number of defects and affects its properties. The process begins by bathing the graphite in a solvent for intercalation. The process can be tracked by monitoring the solution’s transparency with an LED and photodiode. [130][131]
Graphene has been prepared by using a sugar (e.g. glucose, sugar, fructose, etc.) This substrate-free "bottom-up" synthesis is safer, simpler and more environmentally friendly than exfoliation. The method can control thickness, ranging from monolayer to multilayers, which is known as "Tang-Lau Method".[132]
Epitaxial graphene may be coupled to surfaces weakly enough (by Van der Waals forces) to retain the two dimensional electronic band structure of isolated graphene.[133] Heating silicon carbide (SiC) to high temperatures (1100 °C) under low pressures (c. 10−6 torr) reduces it to graphene.[134][135][136][137][138][139] A normal silicon wafer coated with a layer of germanium (Ge) dipped in dilute hydrofluoric acid strips the naturally forming germanium oxide groups, creating hydrogen-terminated germanium. CVD can coat that with graphene.[140][141] A two-step CVD process is shown to grow graphene directly on TiO2 crystals or exfoliated TiO2 nanosheets without using a metal catalyst.[142] The atomic structure of metal substrates including ruthenium,[143] iridium,[144] nickel[145] and copper has been used as substrates for graphene production.[146] Commercial copper foils have been used for graphene production, reducing substrate costs by 100-fold.[147][16] Gaseous catalyst-assisted CVD [148] paves the way for synthesizing high-quality graphene for device applications while avoiding the transfer process. Gram quantities were produced by the reduction of ethanol by sodium metal, followed by pyrolysis and washing with water.[149] Growing graphene in an industrial resistive-heating cold wall CVD system was claimed to produce graphene 100 times faster than conventional CVD systems, cut costs by 99% and produce material with enhanced electronic qualities.[150][151] Experiments with precise control of process parameters during cold wall CVD provided conclusive insight into the classical surface-mediated two dimensional nucleation and growth mechanism of graphene grown using catalytic CVD under conditions sought out in the semiconductor industry.[152][153]
In applications where the thickness and packing density of the graphene layer need to be carefully controlled, the Langmuir-Blodgett method has been used to produce single layer films of graphene and graphene oxide which can then be reduced to graphene.[154][155][156] Some of the benefits of Langmuir-Blodgett deposition include accurate control over the layered architecture of the graphene, that the layer-by-layer deposition process allows assembling any combination of thin carbon layers on a substrates, and that the assembly process is performed at room temperature and produces greater throughputs while being amenable to automation and mass production.[157]
Chemistry
Graphene is the only form of carbon (or solid material) in which every atom is available for chemical reaction from two sides (due to the 2D structure). Atoms at the edges of a graphene sheet have special chemical reactivity. Graphene has the highest ratio of edge atoms of any allotrope. Defects within a sheet increase its chemical reactivity.[158] The onset temperature of reaction between the basal plane of single-layer graphene and oxygen gas is below 260 °C (530 K).[159] Graphene combusts at 350 °C (620 K).[160] Graphene is commonly modified with oxygen- and nitrogen-containing functional groups and analyzed by infrared spectroscopy and X-ray photoelectron spectroscopy. However, determination of structures of graphene with oxygen-[161] and nitrogen-[162] functional groups requires the structures to be well controlled.Contrary to the ideal 2D structure of graphene, chemical applications of graphene need either structural or chemical irregularities, as perfectly flat graphene is chemically inert.[163] In other words, the definition of an ideal graphene is different in chemistry and physics.
Graphene placed on a soda-lime glass (SLG) substrate under ambient conditions exhibited spontaneous n-doping (1.33 × 1013 e/cm2) via surface-transfer. On p-type copper indium gallium diselenide (CIGS) semiconductor itself deposited on SLG n-doping reached 2.11 × 1013 e/cm2.[164]
Various graphene derivatives, e.g., cyanographene and graphene acid, can be prepared via elegant chemistry of fluorographene. Cyanographene and graphene acid have high degree of functionalization (~15%), open band gap and are hydrophilic providing stable colloids in water.
Potential applications

(a) The typical structure of a touch sensor in a touch panel. (Image
courtesy of Synaptics, Incorporated.) (b) An actual example of 2D Carbon
Graphene Material Co., Ltd's graphene transparent conductor-based
touchscreen that is employed in (c) a commercial smartphone.
Graphene is a transparent and flexible conductor that holds promise for various material/device applications, including solar cells,[165] light-emitting diodes (LED), touch panels and smart windows or phones.[166] Graphene has also been used in other fundamental electronic devices, such as capacitors and Field Effect Transistors (FETs), in which it can act as an atomically thin channel. In the same framework, fluorine-doped graphene has shown to have insulating properties and it can be used as a passivation layer in graphene FETs, leading to a substantial increase in carrier mobility.[1]
Other early commercial uses of graphene include fillers such as a graphene-infused printer powder.[167]
Graphene supercapacitors serve as energy storage alternative to traditional electrolytic batteries. Among advantages are fast charging, long life span and environmentally friendly production. Graphene supercapacitors produced by Skeleton Technologies have been commercially available since around 2015 and were first used in some specialized applications instead of traditional batteries.[168] By 2017, commercial graphene supercapacitor units were available for industrial power applications, with maximal power output of 1500 kW.[169] In 2016, Adgero announced a regenerative braking system (KERS) for large trucks that employed a graphene-based supercapacitor.[170] In 2016, Henrik Fisker announced development of an electric car that will use Graphene supercapacitor instead of lithium-ion batteries.[171][needs update]
Its low energy density as compared to lithium-ion batteries is being addressed. The planned electric car would target a minimum range of 400 miles (640 km).[171] It has been announced later that the electric car produced by Fisker Inc. will still use lithium-ion batteries, but research in graphene supercapacitors will continue by Nanotech Energy Inc.[172]
BAC's 2016 Mono model is said to be made out of graphene as a first of both a street-legal track car and a production car.[173][174] The first company to use graphene-made structural parts on a production model was Spania GTA, which unveiled a version of its Spano supercar fitted with graphene in 2015.[174][175]
The global market for graphene reached $9 million by 2012 with most sales in the semiconductor, electronics, battery, energy storage or conversion,[176] and composites industries.[177]
Health and safety
The toxicity of graphene has been extensively debated. A review on graphene toxicity summarized the in vitro, in vivo, antimicrobial and environmental effects and highlights the various mechanisms of graphene toxicity.[178] Nanotubes of graphene could reproduce the effects of asbestosis.[179][180] The toxicity of graphene depends on its shape, size, purity, post-production processing steps, oxidative state, functional groups, dispersion state, synthesis methods, route, dose of administration, and exposure times.Graphene nanoribbons, graphene nanoplatelets, and graphene nano–onions are non-toxic at concentrations up to 50 µg/ml. These nanoparticles do not alter the differentiation of human bone marrow stem cells towards osteoblasts (bone) or adipocytes (fat) suggesting that at low doses graphene nanoparticles are safe for biomedical applications.[181] 10 µm few-layered graphene flakes were able to pierce cell membranes in solution. They were observed to enter initially via sharp and jagged points, allowing graphene to enter the cell. The physiological effects of this remain uncertain, and this remains a relatively unexplored field.[182][183]
History
Konstantin Novoselov (left) and Andre Geim (right) at a 2010 Nobel Prize press conference
A lump of graphite, a graphene transistor, and a tape dispenser, a tool that was used for the exfolitation of single-layer graphene from graphite in 2004. Donated to the Nobel Museum in Stockholm by Andre Geim and Konstantin Novoselov in 2010.
The theory of graphene was first explored by Wallace in 1947 as a starting point for understanding the electronic properties of 3D graphite. The emergent massless Dirac equation was first pointed out by Semenoff, DiVincenzo and Mele.[184] The earliest TEM images of few-layer graphite were published by Ruess and Vogt in 1948.[185] An early, detailed study on few-layer graphite dates to 1962 when Boehm and colleagues reported producing monolayer flakes of reduced graphene oxide.[186][187][188][189] Efforts to make thin films of graphite by mechanical exfoliation started in 1990,[190] but nothing thinner than 50 to 100 layers was produced before 2004. Initial attempts to make atomically thin graphitic films employed exfoliation techniques similar to the drawing method. Multilayer samples down to 10 nm in thickness were obtained.[25]
One of the first patents pertaining to the production of graphene was filed in October 2002 and granted in 2006.[191] Two years later, in 2004 Geim and Novoselov extracted single-atom-thick crystallites from bulk graphite[8] and transferred them onto thin silicon dioxide (SiO
2) on a silicon wafer,[192] which electrically isolated the graphene. The cleavage technique led directly to the first observation of the anomalous quantum Hall effect in graphene,[38][193] which provided direct evidence of graphene's theoretically predicted Berry's phase of massless Dirac fermions. The effect was reported by Geim's group and by Kim and Zhang, whose papers appeared in Nature in 2005.[38][193] Geim and Novoselov received awards for their pioneering research on graphene, notably the 2010 Nobel Prize in Physics.[194]
In 2013, the European Commission funded the large-scale research project Graphene Flagship with a total budget of €1 billion, involving 150 partner organizations.
Commercialization of graphene proceeded rapidly once commercial scale production was demonstrated.[195][196][197] By 2017, 13 years after creation of the first laboratory graphene electronic device, an integrated graphene electronics chip was produced commercially and marketed to pharmaceutical researchers by Nanomedical Diagnostics in San Diego.[198]