The effects of climate change span the physical environment, ecosystems and human societies. It also includes the economic and social changes which stem from living in a warmer world. Human-caused climate change is one of the threats to sustainability.
Many physical impacts of climate change are already visible, including extreme weather events, glacier retreat, changes in the timing of seasonal events (e.g., earlier flowering of plants), sea level rise, and declines in Arctic sea ice extent. The ocean has taken up between 20 and 30% of human-induced atmospheric carbon dioxide since the 1980s, leading to ocean acidification. The ocean is also warming and since 1970 has absorbed more than 90% of the excess heat in the climate system.
Climate change has already impacted ecosystems and humans. In combination with climate variability, it makes food insecurity worse in many places and puts pressure on fresh water supply. This, in combination with extreme weather events, leads to negative effects on human health. Climate change has also contributed to desertification and land degradation in many regions of the world. This has implications for livelihoods as many people are dependent on land for food, feed, fibre, timber and energy. Rising temperatures, changing precipitation patterns and the increase in extreme events threaten development because of negative effects on economic growth in developing countries. Climate change already contributes to migration in different parts of the world.
The future impact of climate change depends on the extent to which nations implement prevention efforts, reduce greenhouse gas emissions, and adapt to unavoidable climate change effects. Much of the policy debate concerning climate change mitigation has been framed by projections for the twenty-first century. The focus on a limited time window obscures some of the problems associated with climate change. Policy decisions made in the next few decades will have profound impacts on the global climate, ecosystems and human societies, not just for this century, but for the next millennia, as near-term climate change policies significantly affect long-term climate change impacts.
Stringent mitigation policies might be able to limit global warming (in 2100) to around 2 °C or below, relative to pre-industrial levels. Without mitigation, increased energy demand and the extensive use of fossil fuels may lead to global warming of around 4 °C. With higher magnitudes of global warming, societies and ecosystems will likely encounter limits to how much they can adapt.
Observed and future warming
Global warming refers to the long-term rise in the average temperature of the Earth's climate system. It is a major aspect of climate change, and has been demonstrated by the instrumental temperature record which shows global warming of around 1 °C since the pre-industrial period, although the bulk of this (0.9 °C) has occurred since 1970. A wide variety of temperature proxies together prove that the 20th century was the hottest recorded in the last 2,000 years. Compared to climate variability in the past, current warming is also more globally coherent, affecting 98% of the planet. The impact on the environment, ecosystems, the animal kingdom, society and humanity depends on how much more the Earth warms.
The Intergovernmental Panel on Climate Change (IPCC) Fifth Assessment Report concluded, "It is extremely likely that human influence has been the dominant cause of the observed warming since the mid-20th century." This has been brought about primarily through the burning of fossil fuels which has led to a significant increase in the concentration of GHGs in the atmosphere.
Emission scenarios
Individual consumers, corporate decision makers, the fossil fuel industries, government responses and the extent to which different countries agree to cooperate all have a profound impact on how much greenhouse gases the worlds emits. As the crisis and modelling techniques have evolved, the IPCC and other climate scientists have tried a number of different tools to estimate likely greenhouse gas emissions in the future.
Representative Concentration Pathways (RCPs) were based on possible differences in radiative forcing occurring in the next 100 years but do not include socioeconomic "narratives" to go alongside them. Another group of climate scientists, economists and energy system modellers took a different approach known as Shared Socioeconomic Pathways (SSPs); this is based on how socioeconomic factors such as population, economic growth, education, urbanisation and the rate of technological development might change over the next century. The SSPs describe five different trajectories which describe future climactic developments in the absence of new environmental policies beyond those in place today. They also explore the implications of different climate change mitigation scenarios.
Warming projections
The range in temperature projections partly reflects the choice of emissions scenario, and the degree of "climate sensitivity". The projected magnitude of warming by 2100 is closely related to the level of cumulative emissions over the 21st century (i.e. total emissions between 2000 and 2100). The higher the cumulative emissions over this time period, the greater the level of warming is projected to occur. Climate sensitivity reflects uncertainty in the response of the climate system to past and future GHG emissions. Higher estimates of climate sensitivity lead to greater projected warming, while lower estimates lead to less projected warming.
The IPCC's Fifth Report,
states that relative to the average from year 1850 to 1900, global
surface temperature change by the end of the 21st century is likely to
exceed 1.5 °C and may well exceed 2 °C for all RCP scenarios
except RCP2.6. It is likely to exceed 2 °C for RCP6.0 and RCP8.5, and
more likely than not to exceed 2 °C for RCP4.5. The pathway with the
highest greenhouse gas emissions, RCP8.5, will lead to a temperature
increase of about 4.3˚C by 2100. Warming will continue beyond 2100 under all RCP scenarios except RCP2.6. Even if emissions were drastically reduced overnight, the warming process is irreversible because CO
2
takes hundreds of years to break down, and global temperatures will
remain close to their highest level for at least the next 1,000 years.
Mitigation policies currently in place will result in about 3.0 °C warming above pre-industrial levels. However, if current plans are not actually implemented, global warming is expected to reach 4.1 °C to 4.8 °C by 2100. There is a substantial gap between national plans and commitments and actual actions so far taken by governments around the world.
Warming in context of Earth's past
One of the methods scientists use to predict the effects of human-caused climate change, is to investigate past natural changes in climate. Scientists have used various "proxy" data to assess changes in Earth's past climate or paleoclimate. Sources of proxy data include historical records such as tree rings, ice cores, corals, and ocean and lake sediments. The data shows that recent warming has surpassed anything in the last 2,000 years.
By the end of the 21st century, temperatures may increase to a level not experienced since the mid-Pliocene, around 3 million years ago. At that time, mean global temperatures were about 2–4 °C warmer than pre-industrial temperatures, and the global mean sea level was up to 25 meters higher than it is today.
Physical impacts
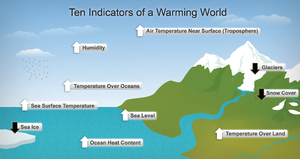
A broad range of evidence shows that the climate system has warmed. Evidence of global warming is shown in the graphs (below right) from the US National Oceanic and Atmospheric Administration (NOAA). Some of the graphs show a positive trend, e.g., increasing temperature over land and the ocean, and sea level rise. Other graphs show a negative trend, such as decreased snow cover in the Northern Hemisphere, and declining Arctic sea ice, both of which are indicative of global warming. Evidence of warming is also apparent in living (biological) systems such as changes in distribution of flora and fauna towards the poles.
Human-induced warming could lead to large-scale, abrupt and/or irreversible changes in physical systems. An example of this is the melting of ice sheets, which contributes to sea level rise and will continue for thousands of years. The probability of warming having unforeseen consequences increases with the rate, magnitude, and duration of climate change.
Effects on weather
Global warming leads to an increase in extreme weather events such as heat waves, droughts, cyclones, blizzards and rainstorms. Such events will continue to occur more often and with greater intensity. Scientists have not only determined that climate change is responsible for trends in weather patterns, some individual extreme weather events have also directly be attributed to climate change.
Precipitation
Higher temperatures lead to increased evaporation and surface drying. As the air warms, its water-holding capacity also increases, particularly over the oceans. In general the air can hold about 7% more moisture for every 1 °C of temperature rise. In the tropics, there's more than a 10% increase in precipitation for a 1 °C increase in temperature. Changes have already been observed in the amount, intensity, frequency, and type of precipitation. Widespread increases in heavy precipitation have occurred even in places where total rain amounts have decreased.
Projections of future changes in precipitation show overall increases in the global average, but with substantial shifts in where and how precipitation falls. Projections suggest a reduction in rainfall in the subtropics, and an increase in precipitation in subpolar latitudes and some equatorial regions. In other words, regions which are dry at present will in general become even drier, while regions that are currently wet will in general become even wetter. Although increased rainfall will not occur everywhere, models suggest most of the world will have a 16–24% increase in heavy precipitation intensity by 2100.
Temperatures
As described in the first section, global temperatures have risen by 1 °C and are expected to rise further in the future. Over most land areas since the 1950s, it is very likely that at all times of year both days and nights have become warmer due to human activities. Night-time temperatures have increased a faster rate than daytime temperatures. In the U.S. since 1999, two warm weather records have been set or broken for every cold one.
Future climate change will include more very hot days and fewer very cold days. The frequency, length and intensity of heat waves will very likely increase over most land areas. Higher growth in anthropogenic GHG emissions would cause more frequent and severe temperature extremes.
Heat waves
Global warming boosts the probability of extreme weather events such as heat waves where the daily maximum temperature exceeds the average maximum temperature by 5 °C (9 °F) for more than five consecutive days.
In the last 30–40 years, heat waves with high humidity have become more frequent and severe. Extremely hot nights have doubled in frequency. The area in which extremely hot summers are observed has increased 50–100 fold. These changes are not explained by natural variability, and are attributed by climate scientists to the influence of anthropogenic climate change. Heat waves with high humidity pose a big risk to human health while heat waves with low humidity lead to dry conditions that increase wildfires. The mortality from extreme heat is larger than the mortality from hurricanes, lightning, tornadoes, floods, and earthquakes together.
Tropical cyclones
Global warming not only causes changes in tropical cyclones, it may also make some impacts from them worse via sea level rise. The intensity of tropical cyclones (hurricanes, typhoons, etc.) is projected to increase globally, with the proportion of Category 4 and 5 tropical cyclones increasing. Furthermore, the rate of rainfall is projected to increase, but trends in the future frequency on a global scale are not yet clear. Changes in tropical cyclones will probably vary by region.
On land
In the year 2019 the Intergovernmental Panel on Climate Change issued a Special Report on Climate Change and Land. The main statements of the report include:
- Humans affect 70% of the ice free land, that play a key role in supplying the needs of humans and in the climate system.
- The global food supply have raised what increased GHG emission, but 25% - 30% of the food is lost, 2 billion adults suffer from overweight while 821 million people suffer from hunger.
- The rate of soil erosion is 10 - 20 times higher than the rate of soil accumulation in agricultural areas that use no-till farming. In areas with tilling it is 100 times higher. Climate Change increases land degradation and desertification.
- In the years 1960 - 2013 the area of drylands in drought, increased by 1% per year.
- In the year 2015 around 500 million people lived in areas that was impacted by desertification in the years 1980s - 2000s.
- People who live in the areas affected by land degradation and desertification are "increasingly negatively affected by climate change".
Climate change will also cause soils to warm. In turn, this could cause the soil microbe population size to dramatically increase 40–150%. Warmer conditions would favor growth of certain bacteria species, shifting the bacterial community composition. Elevated carbon dioxide would increase the growth rates of plants and soil microbes, slowing the soil carbon cycle and favoring oligotrophs, which are slower-growing and more resource efficient than copiotrophs.
Flooding
Warmer air holds more water vapor. When this turns to rain, it tends to come in heavy downpours potentially leading to more floods. A 2017 study found that peak precipitation is increasing between 5 and 10% for every one degree Celsius increase. In the United States and many other parts of the world there has been a marked increase in intense rainfall events which have resulted in more severe flooding. Estimates of the number of people at risk of coastal flooding from climate-driven sea-level rise varies from 190 million, to 300 million or even 640 million in a worst-case scenario related to the instability of the Antarctic ice sheet. the Greenland ice sheet is estimated to have reached a point of no return, continuing to melt even if warming stopped. Over time that would submerge many of the world's coastal cities including low-lying islands, especially combined with storm surges and high tides.
Drought
Climate change affects multiple factors associated with droughts, such as how much rain falls and how fast the rain evaporates again. It is set to increase the severity and frequency of droughts around much of the world. Due to limitations on how much data is available about drought in the past, it is often impossible to confidently attribute droughts to human-induced climate change. Some areas however, such as the Mediterranean and California, already show a clear human signature. Their impacts are aggravated because of increased water demand, population growth, urban expansion, and environmental protection efforts in many areas.
Wildfires
Prolonged periods of warmer temperatures typically cause soil and underbrush to be drier for longer periods, increasing the risk of wildfires. Hot, dry conditions increase the likelihood that wildfires will be more intense and burn for longer once they start. In California, summer air temperature have increased by over 3.5 °F such that the fire season has lengthened by 75 days over previous decades. As a result, since the 1980s, both the size and ferocity of fires in California have increased. Since the 1970s, the size of the area burned has increased fivefold.
In Australia, the annual number of hot days (above 35 °C) and very hot days (above 40 °C) has increased significantly in many areas of the country since 1950. The country has always had bushfires but in 2019, the extent and ferocity of these fires increased dramatically. For the first time catastrophic bushfire conditions were declared for Greater Sydney. New South Wales and Queensland declared a state of emergency but fires were also burning in South Australia and Western Australia.
Cryosphere
The cryosphere is made up of those parts of the planet which are so cold, they are frozen and covered by snow or ice. This includes ice and snow on land such as the continental ice sheets in Greenland and Antarctica, as well as glaciers and areas of snow and permafrost; and ice found on water including frozen parts of the ocean, such as the waters surrounding Antarctica and the Arctic. The cryosphere, especially the polar regions, is extremely sensitive to changes in global climate.
The Intergovernmental Panel on Climate Change issued a Special Report on the Ocean and Cryosphere in a Changing Climate. According to the report climate change caused a massive melting of glaciers, ice sheets, snow and permafrost with generally negative effects on ecosystems and humans. Indigenous knowledge helped to adapt to those effects.
Arctic sea ice began to decline at the beginning of the twentieth century but the rate is accelerating. Since 1979, satellite records indicate the decline in summer sea ice coverage has been about 13% per decade. The thickness of sea ice has also decreased by 66% or 2.0 m over the last six decades with a shift from permanent ice to largely seasonal ice cover. While ice-free summers are expected to be rare at 1.5 °C degrees of warming, they are set to occur at least once every decade at a warming level of 2.0 °C.
Since the beginning of the twentieth century, there has also been a widespread retreat of alpine glaciers, and snow cover in the Northern Hemisphere. During the 21st century, glaciers and snow cover are projected to continue their retreat in almost all regions. The melting of the Greenland and West Antarctic ice sheets will continue to contribute to sea level rise over long time-scales.
Oceans
Global warming is projected to have a number of effects on the oceans. Ongoing effects include rising sea levels
due to thermal expansion and melting of glaciers and ice sheets, and
warming of the ocean surface, leading to increased temperature
stratification.
Other possible effects include large-scale changes in ocean
circulation. The oceans also serve as a sink for carbon dioxide, taking
up much that would otherwise remain in the atmosphere, but increased
levels of CO
2 have led to ocean acidification. Furthermore, as the temperature of the oceans increases, they become less able to absorb excess CO
2. The oceans have also acted as a sink in absorbing extra heat from the atmosphere.
According to a Special Report on the Ocean and Cryosphere in a Changing Climate published by the Intergovernmental Panel on Climate Change, climate change has different impacts on the oceans, including an increase in marine heatwaves, shift in species distribution, ocean deoxygenation.
The decline in mixing of the ocean layers piles up warm water near the surface while reducing cold, deep water circulation. The reduced up and down mixing enhanced global warming. Furthermore, energy available for tropical cyclones and other storms is expected to increase, nutrients for fish in the upper ocean layers are set to decrease, as well as the capacity of the oceans to store carbon.
Sea Ice
Sea ice reflects 50% to 70% of the incoming solar radiation, while 6% of the incoming solar engery is reflected by the ocean. With less solar energy, the sea ice absorbs and holds the surface colder, which can be a positive feedback toward climate change.
Oxygen depletion
Warmer water cannot contain as much oxygen as cold water, so heating is expected to lead to less oxygen in the ocean. Other processes also play a role: stratification may lead to increases in respiration rates of organic matter, further decreasing oxygen content. The ocean has already lost oxygen, throughout the entire water column and oxygen minimum zones are expanding worldwide. This has adverse consequences for ocean life.
Ocean heat uptake
Oceans have taken up over 90% of the excess heat accumulated on Earth due to global warming. The warming rate varies with depth: at a depth of a thousand metres the warming occurs at a rate of almost 0.4 °C per century (data from 1981 to 2019), whereas the warming rate at two kilometres depth is only half. The increase in ocean heat content is much larger than any other store of energy in the Earth's heat balance and accounts for more than 90% of the increase in heat content of the Earth system, and has accelerated in the 1993–2017 period compared to 1969–1993. In 2019 a paper published in the journal Science found the oceans are heating 40% faster than the IPCC predicted just five years before.
As well as having effects on ecosystems (e.g. by melting sea ice affecting algae that grow on its underside), warming reduces the ocean's ability to absorb CO
2. It is likely that the oceans warmed faster between 1993 and 2017 compared to the period starting in 1969.
Sea level rise
The IPCC's Special Report on the Ocean and Cryosphere concluded that global mean sea level rose by 0.16 metres between 1901 and 2016. The rate of sea level rise since the industrial revolution in the 19th century has been larger than the rate during the previous two thousand years.
Global sea level rise is accelerating, rising 2.5 times faster between 2006 and 2016 than it did during the 20th century. Two main factors contribute to the rise. The first is thermal expansion: as ocean water warms, it expands. The second is from the melting of land-based ice in glaciers and ice sheets due to global warming. Prior to 2007, thermal expansion was the largest component in these projections, contributing 70–75% of sea level rise. As the impact of global warming has accelerated, melting from glaciers and ice sheets has become the main contributor.
Even if emission of greenhouse gases stops overnight, sea level rise will continue for centuries to come. In 2015, a study by Professor James Hansen of Columbia University and 16 other climate scientists said a sea level rise of three metres could be a reality by the end of the century. Another study by scientists at the Royal Netherlands Meteorological Institute in 2017 using updated projections of Antarctic mass loss and a revised statistical method also concluded that, although it was a low probability, a three-metre rise was possible. Rising sea levels will put hundreds of millions of people at risk in low-lying coastal areas in countries such as China, Bangladesh, India and Vietnam.
Wildlife and nature
Recent warming has strongly affected natural biological systems. Species worldwide are moving poleward to colder areas. On land, species move to higher elevations, whereas marine species find colder water at greater depths. Of the drivers with the biggest global impact on nature, climate change ranks third over the five decades before 2020, with only change in land use and sea use, and direct exploitation of organisms having a greater impact.
The impacts of climate change in nature and nature's contributions to humans are projected to become more pronounced in the next few decades. Examples of climatic disruptions include fire, drought, pest infestation, invasion of species, storms, and coral bleaching events. The stresses caused by climate change, added to other stresses on ecological systems (e.g. land conversion, land degradation, harvesting, and pollution), threaten substantial damage to or complete loss of some unique ecosystems, and extinction of some critically endangered species. Key interactions between species within ecosystems are often disrupted because species from one location do not move to colder habitats at the same rate, giving rise to rapid changes in the functioning of the ecosystem.
Terrestrial and wetland systems
Climate change has been estimated to be a major driver of biodiversity loss in cool conifer forests, savannas, mediterranean-climate systems, tropical forests, and the Arctic tundra. In other ecosystems, land-use change may be a stronger driver of biodiversity loss, at least in the near-term. Beyond the year 2050, climate change may be the major driver for biodiversity loss globally. Climate change interacts with other pressures such as habitat modification, pollution and invasive species. Interacting with these pressures, climate change increases extinction risk for a large fraction of terrestrial and freshwater species. Between 1% and 50% of species in different groups were assessed to be at substantially higher risk of extinction due to climate change.
Ocean ecosystems
Warm water coral reefs are very sensitive to global warming and ocean acidification. Coral reefs provide a habitat for thousands of species and ecosystem services such as coastal protection and food. The resilience of reefs can be improved by curbing local pollution and overfishing, but most warm water coral reefs will disappear even if warming is kept to 1.5 °C. Coral reefs are not the only framework organisms, organisms that build physical structures that form habitats for other sea creatures, affected by climate change: mangroves and seagrass are considered to be at moderate risk for lower levels of global warming according to a literature assessment in the Special Report on the Ocean and Cryosphere in a Changing Climate. Marine heatwaves have seen an increased frequency and have widespread impacts on life in the oceans, such as mass dying events. Harmful algae blooms have increased in response to warming waters, ocean deoxygenation and eutrophication. Between one-quarter and one-third of our fossil fuel emissions are consumed by the earth's oceans and are now 30 percent more acidic than they were in pre-industrial times. This acidification poses a serious threat to aquatic life, particularly creatures such as oysters, clams, and coral with calcified shells or skeletons.
Regional effects
Regional effects of global warming vary in nature. Some are the result of a generalised global change, such as rising temperature, resulting in local effects, such as melting ice. In other cases, a change may be related to a change in a particular ocean current or weather system. In such cases, the regional effect may be disproportionate and will not necessarily follow the global trend.
There are three major ways in which global warming will make changes to regional climate: melting or forming ice, changing the hydrological cycle (of evaporation and precipitation) and changing currents in the oceans and air flows in the atmosphere. The coast can also be considered a region, and will suffer severe impacts from sea level rise.
The Arctic, Africa, small islands, Asian megadeltas and the Middle East are regions that are likely to be especially affected by climate change. Low-latitude, less-developed regions are at most risk of experiencing negative impacts due to climate change. Developed countries are also vulnerable to climate change. For example, developed countries will be negatively affected by increases in the severity and frequency of some extreme weather events, such as heat waves.
Projections of climate changes at the regional scale do not hold as high a level of scientific confidence as projections made at the global scale. It is, however, expected that future warming will follow a similar geographical pattern to that seen already, with the greatest warming over land and high northern latitudes, and least over the Southern Ocean and parts of the North Atlantic Ocean. Land areas warm faster than ocean, and this feature is even stronger for extreme temperatures. For hot extremes, regions with the most warming include Central and Southern Europe and Western and Central Asia.
On humans
The effects of climate change, in combination with the sustained increases in greenhouse gas emissions, have led scientists to characterize it as a climate emergency. Some climate researchers and activists have called it an existential threat to civilization. Some areas may become too hot for humans to live in while people in some areas may experience displacement triggered by flooding and other climate change related disasters.
The vulnerability and exposure of humans to climate change varies from one economic sector to another and will have different impacts in different countries. Wealthy industrialised countries, which have emitted the most CO2, have more resources and so are the least vulnerable to global warming. Economic sectors that are likely to be affected include agriculture, human health, fisheries, forestry, energy, insurance, financial services, tourism, and recreation. The quality and quantity of freshwater will likely be affected almost everywhere. Some people may be particularly at risk from climate change, such as the poor, young children and the elderly. According to the World Health Organization, between 2030 and 2050, "climate change is expected to cause about 250,000 additional deaths per year." As global temperatures increase, so does the number of heat stress, heatstroke, and cardiovascular and kidney disease deaths and illnesses. Air pollution generated by fossil fuel combustion is both a major driver of global warming and – in parallel and for comparison – the cause of a large number of annual deaths with some estimates as high as 8.7 million excess deaths during 2018. It may be difficult to predict or attribute deaths to anthropogenic global warming or its particular drivers as many effects – such as possibly contributing to human conflict and socioeconomic disruptions – and their mortality impacts could be highly indirect or hard to evaluate.
Food security
Climate change will impact agriculture and food production around the world due to the effects of elevated CO2 in the atmosphere; higher temperatures; altered precipitation and transpiration regimes; increased frequency of extreme events; and modified weed, pest, and pathogen pressure. Climate change is projected to negatively affect all four pillars of food security: not only how much food is available, but also how easy food is to access (prices), food quality and how stable the food system is.
Food availability
As of 2019, negative impacts have been observed for some crops in low-latitudes (maize and wheat), while positive impacts of climate change have been observed in some crops in high-latitudes (maize, wheat, and sugar beets). Using different methods to project future crop yields, a consistent picture emerges of global decreases in yield. Maize and soybean decrease with any warming, whereas rice and wheat production might peak at 3 °C of warming.
In many areas, fisheries have already seen their catch decrease because of global warming and changes in biochemical cycles. In combination with overfishing, warming waters decrease the maximum catch potential. Global catch potential is projected to reduce further in 2050 by less than 4% if emissions are reduced strongly, and by about 8% for very high future emissions, with growth in the Arctic Ocean.
Other aspects of food security
Climate change impacts depend strongly on projected future social and economic development. As of 2019, an estimated 831 million people are undernourished. Under a high emission scenario (RCP6.0), cereals are projected to become 1-29% more expensive in 2050 depending on the socioeconomic pathway, particularly affecting low-income consumers. Compared to a no climate change scenario, this would put between 1-181 million extra people at risk of hunger.
While CO
2
is expected to be good for crop productivity at lower temperatures, it
does reduce the nutritional values of crops, with for instance wheat
having less protein and less of some minerals.
It is difficult to project the impact of climate change on utilization
(protecting food against spoilage, being healthy enough to absorb
nutrients, etc.) and on volatility of food prices. Most models projecting the future do indicate that prices will become more volatile.
Droughts result in crop failures and the loss of pasture for livestock.
Water security
A number of climate-related trends have been observed that affect water resources. These include changes in precipitation, the cryosphere and surface waters (e.g., changes in river flows). Observed and projected impacts of climate change on freshwater systems and their management are mainly due to changes in temperature, sea level and precipitation variability. Changes in temperature are correlated with variability in precipitation because the water cycle is reactive to temperature. Temperature increases change precipitation patterns. Excessive precipitation leads to excessive sediment deposition, nutrient pollution, and concentration of minerals in aquifers.
The rising global temperature will cause sea level rise and will extend areas of salinization of groundwater and estuaries, resulting in a decrease in freshwater availability for humans and ecosystems in coastal areas. The rising sea level will push the salt gradient into freshwater deposits and will eventually pollute freshwater sources. The 2014 fifth IPCC assessment report concluded that:
- Water resources are projected to decrease in most dry subtropical regions and mid-latitudes, but increase in high latitudes. As streamflow becomes more variable, even regions with increased water resources can experience additional short-term shortages.
- Per degree warming, a model average of 7% of the world population is expected to have at least 20% less renewable water resource.
- Climate change is projected to reduce water quality before treatment. Even after conventional treatments, risks remain. The quality reduction is a consequence of higher temperatures, more intense rainfall, droughts and disruption of treatment facilities during floods.
- Droughts that stress water supply are expected to increase in southern Europe and the Mediterranean region, central Europe, central and southern North America, Central America, northeast Brazil, and southern Africa.
Health
Humans are exposed to climate change through changing weather patterns (temperature, precipitation, sea-level rise and more frequent extreme events) and indirectly through changes in water, air and food quality and changes in ecosystems, agriculture, industry and settlements and the economy. Air pollution, wildfires, and heat waves caused by global warming have significantly affected human health, and in 2007, the World Health Organization estimated 150,000 people were being killed by climate-change-related issues every year.
A study by the World Health Organization concluded that climate change was responsible for 3% of diarrhoea, 3% of malaria, and 3.8% of dengue fever deaths worldwide in 2004. Total attributable mortality was about 0.2% of deaths in 2004; of these, 85% were child deaths. The effects of more frequent and extreme storms were excluded from this study.
The human impacts include both the direct effects of extreme weather, leading to injury and loss of life, as well as indirect effects, such as undernutrition brought on by crop failures. Various infectious diseases are more easily transmitted in a warmer climate, such as dengue fever, which affects children most severely, and malaria. Young children are the most vulnerable to food shortages, and together with older people, to extreme heat.
According to a report from the United Nations Environment Programme and International Livestock Research Institute, climate change can facilitate outbreaks of Zoonosis, e.g. diseases that pass from animals to humans. One example of such outbreaks is the COVID-19 pandemic.
A minor further effect are increases of pollen season lengths and concentrations in some regions of the world.
Projections
A 2014 study by the World Health Organization estimated the effect of climate change on human health, but not all of the effects of climate change were included in their estimates. For example, the effects of more frequent and extreme storms were excluded. The report further assumed continued progress in health and growth. Even so, climate change was projected to cause an additional 250,000 deaths per year between 2030 and 2050.
The authors of the IPCC AR4 Synthesis report projected with high confidence that climate change will bring some benefits in temperate areas, such as fewer deaths from cold exposure, and some mixed effects such as changes in range and transmission potential of malaria in Africa. Benefits were projected to be outweighed by negative health effects of rising temperatures, especially in developing countries.
Economic development is an important component of possible adaptation to climate change. Economic growth on its own, however, is not sufficient to insulate the world's population from disease and injury due to climate change. Future vulnerability to climate change will depend not only on the extent of social and economic change, but also on how the benefits and costs of change are distributed in society. For example, in the 19th century, rapid urbanization in western Europe led to health plummeting. Other factors important in determining the health of populations include education, the availability of health services, and public-health infrastructure.
On mental health
In 2018, the American Psychological Association issued a report about the impact of climate change on mental health. It said that "gradual, long-term changes in climate can also surface a number of different emotions, including fear, anger, feelings of powerlessness, or exhaustion". Generally this is likely to have the greatest impact on young people. California social scientist, Renee Lertzman, likens the climate-related stress now affecting teenagers and those in their 20s to Cold War fears that gripped young baby boomers who came of age under the threat of nuclear annihilation. Research has found that although there are heightened emotional experiences linked with acknowledgement and anticipation of climate change and its impact on society, these are inherently adaptive. Furthermore, engaging with these emotional experiences leads to increased resilience, agency, reflective functioning and collective action. Individuals are encouraged to find collective ways of processing their climate related emotional experiences in order to support mental health and well being. A 2018 study found that unusually hot days have profound effects on mental health and that global warming could contribute to approximately 26,000 more suicides in the U.S. by 2050. A study published in April 2020 found that by the end of the 21st century people could be exposed to avoidable indoor CO2 levels of up to 1400 ppm, which would be triple the amount commonly experienced outdoors today and, according to the authors, may cut humans' basic decision-making ability indoors by ~25% and complex strategic thinking by ~50%.
Migration
Gradual but pervasive environmental change and sudden natural disasters both influence the nature and extent of human migration but in different ways.
Slow onset
Slow-onset disasters and gradual environmental erosion such as desertification, reduction of soil fertility, coastal erosion and sea-level rise are likely to induce long term migration. Migration related to desertification and reduced soil fertility is likely to be predominantly from rural areas in developing countries to towns and cities.
Displacement and migration related to sea level rise will mostly affect those who live in cities near the coast. More than 90 US coastal cities are already experiencing chronic flooding and that number is expected to double by 2030. Numerous cities in Europe will be affected by rising sea levels; especially in the Netherlands, Spain and Italy. Coastal cities in Africa are also under threat due to rapid urbanization and the growth of informal settlements along the coast. Low lying Pacific island nations including Fiji, Kiribati, Nauru, Micronesia, the Marshall Islands, the Solomon Islands, Vanuatu, Timor Leste and Tonga are especially vulnerable to rising seas. In July 2019, they issued a declaration "affirming that climate change poses the single greatest threat to the human rights and security of present and future generations of Pacific Island peoples" and stated their lands could become uninhabitable as early as 2030.
The United Nations says there are already 64 million human migrants in the world fleeing wars, hunger, persecution and the effects of global warming. In 2018, the World Bank estimated that climate change will cause internal migration of between 31 and 143 million people as they escape crop failures, water scarcity, and sea level rise. The study only included Sub-Saharan Africa, South Asia, and Latin America.
A 2020 study projects that regions inhabited by a third of the human population could become as hot as the hottest parts of the Sahara within 50 years without a change in patterns of population growth and without migration, unless greenhouse gas emissions are reduced. The projected annual average temperature of above 29 °C for these regions would be outside the "human temperature niche" – a suggested range for climate biologically suitable for humans based on historical data of mean annual temperatures (MAT) – and the most affected regions have little adaptive capacity as of 2020. The following matrix shows their projections for population-sizes outside the "human temperature niche" – and therefore potential emigrants of their regions – in different climate change scenarios and projections of population growth for 2070:
Sudden onset
Sudden-onset natural disasters tend to create mass displacement, which may only be short term. However, Hurricane Katrina demonstrated that displacement can last a long time. Estimates suggest that a quarter of the one million people displaced in the Gulf Coast region by Hurricane Katrina had not returned to their homes five years after the disaster. Mizutori, the U.N. secretary-general's special representative on disaster risk reduction, says millions of people are also displaced from their homes every year as result of sudden-onset disasters such as intense heatwaves, storms and flooding. She says 'climate crisis disasters' are happening at the rate of one a week.
Conflict
A 2013 study found that significant climatic changes were associated with a higher risk of conflict worldwide, and predicted that "amplified rates of human conflict could represent a large and critical social impact of anthropogenic climate change in both low- and high-income countries." Similarly, a 2014 study found that higher temperatures were associated with a greater likelihood of violent crime, and predicted that global warming would cause millions of such crimes in the United States alone during the 21st century. Climate change can worsen conflicts by exacerbating tensions over limited resources like drinking water. Climate change has the potential to cause large population dislocations, which can also lead to conflict.
However, a 2018 study in the journal Nature Climate Change found that previous studies on the relationship between climate change and conflict suffered from sampling bias and other methodological problems. Factors other than climate change are judged to be substantially more important in affecting conflict (based on expert elicitation). These factors include intergroup inequality and low socio-economic development.
Despite these issues, military planners are concerned that global warming is a "threat multiplier". "Whether it is poverty, food and water scarcity, diseases, economic instability, or threat of natural disasters, the broad range of changing climatic conditions may be far reaching. These challenges may threaten stability in much of the world". For example, the onset of the Arab Spring in 2010 was partly the result of a spike in wheat prices following crop losses from the 2010 Russian heat wave.
Economic impact
Economic forecasts of the impact of global warming vary considerably. Researchers have warned that current economic modelling may seriously underestimate the impact of potentially catastrophic climate change, and point to the need for new models that give a more accurate picture of potential damages. Nevertheless, one recent study has found that potential global economic gains if countries implement mitigation strategies to comply with the 2 °C target set at the Paris Agreement are in the vicinity of US$17 trillion per year up to 2100 compared to a very high emission scenario.
Global losses reveal rapidly rising costs due to extreme weather events since the 1970s. Socio-economic factors have contributed to the observed trend of global losses, such as population growth and increased wealth. Part of the growth is also related to regional climatic factors, e.g., changes in precipitation and flooding events. It is difficult to quantify the relative impact of socio-economic factors and climate change on the observed trend. The trend does, however, suggest increasing vulnerability of social systems to climate change.
A 2019 modelling study found that climate change had contributed towards global economic inequality. Wealthy countries in colder regions had either felt little overall economic impact from climate change, or possibly benefited, whereas poor hotter countries very likely grew less than if global warming had not occurred.
The total economic impacts from climate change are difficult to estimate, but increase for higher temperature changes. For instance, total damages are estimated to be 90% less if global warming is limited to 1.5 °C compared to 3.66 °C, a warming level chosen to represent no mitigation. One study found a 3.5% reduction in global GDP by the end of the century if warming is limited to 3 °C, excluding the potential effect of tipping points. Another study noted that global economic impact is underestimated by a factor of two to eight when tipping points are excluded from consideration. In the Oxford Economics high emission scenario, a temperature rise of 2 degrees by the year 2050 would reduce global GDP by 2.5% - 7.5%. By the year 2100 in this case, the temperature would rise by 4 degrees, which could reduce the global GDP by 30% in the worst case.
Abrupt or irreversible changes
Self-reinforcing feedbacks amplify and accelerate climate change. The climate system exhibits threshold behaviour or tipping points when these feedbacks lead parts of the Earth system into a new state, such as the runaway loss of ice sheets or the destruction of too many forests. Tipping points are studied using data from Earth's distant past and by physical modelling. There is already moderate risk of global tipping points at 1 °C above pre-industrial temperatures, and that risk becomes high at 2.5 °C.
Tipping points are "perhaps the most ‘dangerous’ aspect of future climate changes", leading to irreversible impacts on society. Many tipping points are interlinked, so that triggering one may lead to a cascade of effects. A 2018 study states that 45% of environmental problems, including those caused by climate change are interconnected and make the risk of a domino effect bigger.
Amazon rain forest
Rainfall that falls on the Amazon rainforest is recycled when it evaporates back into the atmosphere instead of running off away from the rainforest. This water is essential for sustaining the rainforest. Due to deforestation the rainforest is losing this ability, exacerbated by climate change which brings more frequent droughts to the area. The higher frequency of droughts seen in the first two decades of the 21st century signal that a tipping point from rainforest to savanna might be close.
Greenland and West Antarctic Ice sheets
Future melt of the West Antarctic ice sheet is potentially abrupt under a high emission scenario, as a consequence of a partial collapse. Part of the ice sheet is grounded on bedrock below sea level, making it possibly vulnerable to the self-enhancing process of marine ice sheet instability. A further hypothesis is that marine ice cliff instability would also contribute to a partial collapse, but limited evidence is available for its importance. A partial collapse of the ice sheet would lead to rapid sea level rise and a local decrease in ocean salinity. It would be irreversible on a timescale between decades and millennia.
In contrast to the West Antarctic ice sheet, melt of the Greenland ice sheet is projected to be taking place more gradually over millennia. Sustained warming between 1 °C (low confidence) and 4 °C (medium confidence) would lead to a complete loss of the ice sheet, contributing 7 m to sea levels globally. The ice loss could become irreversible due to a further self-enhancing feedback: the elevation-surface mass balance feedback. When ice melts on top of the ice sheet, the elevation drops. As air temperature is higher at lower altitude, this promotes further melt.
Atlantic Meridional Overturning Circulation
The Atlantic Meridional Overturning Circulation
(AMOC), an important component of the Earth's climate system, is a
northward flow of warm, salty water in the upper layers of the Atlantic and a southward flow of colder water in the deep Atlantic.
Potential impacts associated with AMOC changes include reduced warming
or (in the case of abrupt change) absolute cooling of northern
high-latitude areas near Greenland and north-western Europe, an increased warming of Southern Hemisphere high-latitudes, tropical drying, as well as changes to marine ecosystems, terrestrial vegetation, oceanic CO
2 uptake, oceanic oxygen concentrations, and shifts in fisheries.
According to a 2019 assessment in the IPCC's Special Report on the Ocean and Cryosphere in a Changing Climate it is very likely (greater than 90% probability, based on expert judgement) that the strength of the AMOC will decrease further over the course of the 21st century. Warming is still expected to occur over most of the European region downstream of the North Atlantic Current in response to increasing GHGs, as well as over North America. With medium confidence, the IPCC stated that it is very unlikely (less than 10% probability) that the AMOC will collapse in the 21st century. The potential consequences of such a collapse could be severe.
Irreversible change
Warming commitment to CO
2 concentrations.
If emissions of CO
2 were to be abruptly stopped and no negative emission technologies
deployed, the Earth's climate would not start moving back to its
pre-industrial state. Instead, temperatures would stay elevated at the
same level for several centuries. After about a thousand years, 20% to
30% of human-emitted CO
2
will remain in the atmosphere, not taken up by the ocean or the land,
committing the climate to warming long after emissions have stopped. Pathways that keep global warming under 1.5 °C often rely on large-scale removal of CO
2, which feasibility is uncertain and has clear risks.
Irreversible impacts
There are a number of examples of climate change impacts that may be irreversible, at least over the timescale of many human generations. These include the large-scale singularities such as the melting of the Greenland and West Antarctic ice sheets, and changes to the AMOC. In biological systems, the extinction of species would be an irreversible impact. In social systems, unique cultures may be lost due to climate change. For example, humans living on atoll islands face risks due to sea level rise, sea surface warming, and increased frequency and intensity of extreme weather events.