From Wikipedia, the free encyclopedia
Cannabidiol (CBD) is a phytocannabinoid discovered in 1940. It is one of 113 identified cannabinoids in cannabis plants, along with tetrahydrocannabinol (THC), and accounts for up to 40% of the plant's extract. As of 2019, clinical research on CBD included studies related to anxiety, cognition, movement disorders, and pain, but there is insufficient high-quality evidence that cannabidiol is effective for these conditions.
Cannabidiol can be taken internally in multiple ways, including by inhaling cannabis smoke or vapor, by mouth, and as an aerosol spray into the cheek. It may be supplied as CBD oil containing only CBD as the active ingredient (excluding tetrahydrocannabinol [THC] or terpenes), CBD-dominant hemp extract oil, capsules, dried cannabis, or prescription liquid solution. CBD does not have the same psychoactivity as THC, and may change the effects of THC on the body if both are present. As of 2018, the mechanism of action for its biological effects has not been determined.
In the United States, the cannabidiol drug Epidiolex was approved by the Food and Drug Administration in 2018 for the treatment of two epilepsy disorders. Since cannabis is a Schedule I controlled substance in the United States, other CBD formulations remain illegal under federal law to prescribe for medical use or to use as an ingredient in dietary supplements or other foods.
Medical uses
Cannabidiol is the generic name of the drug and its INN.
Research
As of 2019, there was limited high-quality evidence for cannabidiol having a neurological effect in people.
Epilepsy
In the United States, the FDA has indicated only one brand of prescription cannabidiol called "Epidiolex" for the treatment of seizures associated with Dravet syndrome, Lennox-Gastaut syndrome, or tuberous sclerosis complex in people one year of age and older. While Epidiolex treatment is generally well tolerated, it is associated with minor adverse effects, such as gastrointestinal upset, decreased appetite, lethargy, sleepiness and poor sleep quality.
In the European Union, cannabidiol (Epidyolex) is indicated for
use as adjunctive therapy of seizures associated with Lennox Gastaut
syndrome (LGS) or Dravet syndrome (DS), in conjunction with clobazam, for people two years of age and older.
In 2020, the label for Epidiolex in the US was expanded to include
seizures associated with tuberous sclerosis complex. Epidiolex/Epidyolex
is the first prescription formulation of plant-derived cannabidiol
approved by regulatory bodies in the US and Europe.
Other uses
Research on other uses for cannabidiol includes several neurological disorders, but the findings have not been confirmed to establish such uses in clinical practice. In October 2019, the FDA issued an advisory warning that the effects of CBD during pregnancy or breastfeeding are unknown, indicating that the safety, doses, interactions with other drugs or foods, and side effects of CBD are not clinically defined, and may pose a risk to the mother and infant.
Many claims are made for the therapeutic benefit of cannabidiol
that are not backed by sound evidence. Some claims – for example that
cannabidiol can be used to treat cancer – fall into the realm of pseudoscience.
In 2020, the label for Epidiolex in the US was expanded to
include treatment of seizures associated with tuberous sclerosis
complex.
Non-intoxicating effects
Cannabidiol does not appear to have any intoxicating effects (i.e., "getting high") such as those caused by ∆9-THC in marijuana, but it is under preliminary research for its possible anti-anxiety and anti-psychotic effects.
As the legal landscape and understanding about the differences in
medical cannabinoids unfolds, experts are working to distinguish
"medical marijuana" (with varying degrees of psychotropic effects and
deficits in executive function) from "medical CBD therapies", which
would commonly present as having a reduced or non-psychoactive
side-effect profile.
Various strains of "medical marijuana" are found to have a
significant variation in the ratios of CBD-to-THC and are known to
contain other non-psychotropic cannabinoids. Any psychoactive marijuana, regardless of its CBD content, is derived from the flower (or bud) of the genus Cannabis. As defined by US federal law, non-psychoactive hemp (also commonly termed "industrial hemp"), regardless of its CBD content, is any part of the cannabis plant, whether growing or not, containing a ∆9-tetrahydrocannabinol concentration of no more than 0.3% on a dry-weight basis.
Certain standards are required for legal growing, cultivating, and
producing the hemp plant, but there are no federal standards for quality
being enforced in the hemp industry. Certain state regulations are in
place, but vary state to state.
For instance, the Colorado Industrial Hemp Program registers growers of
industrial hemp and samples crops to verify that the dry-weight THC
concentration does not exceed 0.3%.
Side effects
Research indicates that cannabidiol may reduce adverse effects of THC, particularly those causing intoxication and sedation, but only at high doses. Safety studies of cannabidiol showed it is well tolerated, but may cause tiredness, diarrhea, or changes in appetite as common adverse effects. Epidiolex documentation lists sleepiness, insomnia and poor quality sleep, decreased appetite, diarrhea, and fatigue.
Potential interactions
Laboratory evidence indicated that cannabidiol may reduce THC clearance, increasing plasma concentrations which may raise THC availability to receptors and enhance its effect in a dose-dependent manner. In vitro, cannabidiol inhibited the activity of voltage-dependent sodium and potassium channels, which may affect neural activity.
A recent study using X-ray crystallography showed that CBD binds inside
the sodium channel pore at a novel site at the interface of the
fenestrations and the central hydrophobic cavity of the channel. Binding
at this site blocks the transmembrane-spanning sodium ion translocation
pathway, providing a molecular mechanism for channel inhibition, which
could contribute to a reduced excitability. A small clinical trial reported that CBD partially inhibited the CYP2C-catalyzed hydroxylation of THC to 11-OH-THC. Little is known about potential drug interactions, but CBD mediates a decrease in clobazam metabolism.
Veterinary medicine
Research
The
number of research projects and scientific publications on cannabidiol
and other cannabinoids in pets has surged in the late 2010s.
As of December 2020, there are no hemp-derived, cannabinoid-rich
registered veterinary medicinal products in any of the major regions
(see Legal status across countries).
In the USA and other territories there are, however, numerous
veterinary nutraceutical products available OTC. The lack of clarity in
the regulations governing veterinary hemp food supplements allows for
products of questionable quality to flood the market, which may pose a risk to the wellbeing of pets and owners.
To understand better the benefits of CBD and associated compounds
for the quality of life of animals, companies specialized in CBD
products for animals have been funding research projects.
Canine osteoarthritis
CBD's ability to help regulate the endocannabinoid system
and reduce the release of excitatory neurotransmitters could result in a
retrograde inhibitory signal that lessens chronic pain responses.
Studies in dogs suffering from chronic pain associated with
osteoarthritis showed an increase in level of activity in animals
receiving CBD-rich food supplements.
Epilepsy
From the results seen in humans with drugs such as Epidyolex and Sativex in scientific studies and reviews,
it could be expected that CBD-based products would be helpful to manage
seizures in dogs. However, despite the numerous case reports presented
by veterinary neurologists supporting the benefits of CBD as adjunctive
therapy, as of December 2020, published controlled studies have not
shown a statistically significant decrease in the number of seizures
across the groups receiving CBD. Further research in this area is required before any clear conclusion can be drawn.
Pharmacokinetics
The
oral bioavailability of CBD varies greatly across species and it is
linked to the presentation and the time of administration.
A 24-hour kinetic examination in dogs showed that the absorption of the
cannabidiolic acid (CBDA) does occur, and that this molecule is
absorbed least twice as well as CBD post oral ingestion.
It was found that the major metabolites of CBD in humans (7-OH-CBD
and 7-COOH-CBD) are not prevalent in dogs, while 6-OH-CBD was found to
be the primary metabolite in dogs receiving a CBD-enriched
cannabis-derived herbal extract, suggesting that canine and human CBD metabolic route might be somewhat different.
Safety
Increases in Alkaline Phosphatase (ALP) have been reported in various canine studies.
A 39-week study in dogs evaluating doses of 0, 10, 50, and 100 mg of
CBD/kg reported an increase of up to 8x in the ALP in the 100 mg/kg
group, and of up to 1.5x in Alanine Aminotransferase (ALT) in the
50 mg/kg and 100 mg/kg groups. However, there were no clinical signs
associated with these results.
Pharmacology
Pharmacodynamics
Cannabidiol has low affinity for the cannabinoid CB1 and CB2 receptors, although it can act as an antagonist of CB1/CB2 agonists despite this low affinity. Cannabidiol may be an antagonist of GPR55, a G protein-coupled receptor and putative cannabinoid receptor that is expressed in the caudate nucleus and putamen in the brain. It also may act as an inverse agonist of GPR3, GPR6, and GPR12. CBD has been shown to act as a serotonin 5-HT1A receptor partial agonist. It is an allosteric modulator of the μ- and δ-opioid receptors as well. The pharmacological effects of CBD may involve PPARγ agonism, inhibition of voltage-gated cation channels, and intracellular calcium release.
Pharmacokinetics
The oral bioavailability of cannabidiol is approximately 6% in humans, while its bioavailability via inhalation is 11 to 45% (mean 31%). The elimination half-life of CBD is 18–32 hours. Cannabidiol is metabolized in the liver as well as in the intestines by the cytochrome P450 enzymes CYP2B6, CYP2C19, CYP2D6, CYP2J2, and CYP3A4, and by the isoenzymes UGT1A7, UGT1A9, and UGT2B7, forming a variety of metabolites such as 7-hydroxycannabidiol as well as the 6α- and 6β-hydroxy isomers and derivatives hydroxylated on the alkyl side chain, followed by glucuronidation. CBD may have a wide margin in dosing.
Pharmaceutical preparations
Nabiximols (brand name Sativex),
an oromucosal spray made of a complex botanical mixture containing
cannabidiol (CBD), delta-9-tetrahydrocannabinol (THC), and additional
cannabinoid and non-cannabinoid constituents from cannabis sativa
plants, was approved by Health Canada in 2005 to treat central neuropathic pain in multiple sclerosis, and in 2007 for cancer-related pain.
In New Zealand, Sativex is "approved for use as an add-on treatment for
symptom improvement in people with moderate to severe spasticity due to
multiple sclerosis who have not responded adequately to other
anti-spasticity medication."
Epidiolex is an orally administered cannabidiol solution. It was approved in 2018 by the US Food and Drug Administration (FDA) for treatment of two rare forms of childhood epilepsy, Lennox-Gastaut syndrome and Dravet syndrome, and seizures associated with tuberous sclerosis complex. In the US, it is approved in these indications for patients one year of age and older.
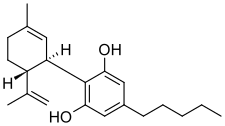
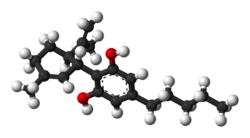
Chemistry
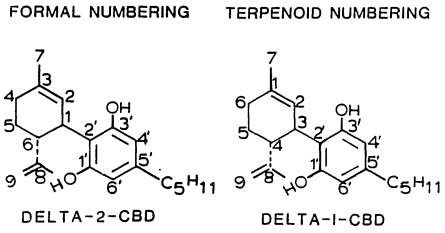
At room temperature, cannabidiol is a colorless crystalline solid. In strongly basic media and the presence of air, it is oxidized to a quinone. Under acidic conditions it cyclizes to THC, which also occurs during pyrolysis, but not during combustion (smoking). The synthesis of cannabidiol has been accomplished by several research groups.
|
|
| Possible mechanism of intramolecular cyclization of CBD to Δ9-THC
|
Biosynthesis
Cannabidiol and THC biosynthesis
Cannabis produces CBD-carboxylic acid through the same metabolic pathway as THC, until the next to last step, where CBDA synthase performs catalysis instead of THCA synthase.
Isomerism
Cannabidiol's 7 double bond isomers and their 30 stereoisomers
History
Efforts to isolate the active ingredients in cannabis were made in the 19th century. Cannabidiol was studied in 1940 from Minnesota wild hemp and Egyptian Cannabis indica resin. The chemical formula of CBD was proposed from a method for isolating it from wild hemp. Its structure and stereochemistry were determined in 1963.
Plant breeding
Selective breeding of cannabis plants has expanded and diversified as commercial and therapeutic markets develop.
Some growers in the US succeeded in lowering the proportion of
CBD-to-THC to accommodate customers who preferred varietals that were
more mind-altering due to the higher THC and lower CBD content.
In the US, hemp is classified by the federal government as cannabis
containing no more than 0.3% THC by dry weight. This classification was
established in the 2018 Farm Bill and was refined to include
hemp-sourced extracts, cannabinoids, and derivatives in the definition
of hemp.
Society and culture
Foods and beverages
An example of beverages claiming to contain CBD in a Los Angeles grocery
Food and beverage products containing cannabidiol were widely marketed in the United States as early as 2017.
Hemp seed ingredients which do not naturally contain THC or CBD (but
which may be contaminated with trace amounts on the outside during
harvesting) were declared by the US Food and Drug Administration (FDA) as Generally recognized as safe
(GRAS) in December 2018. CBD itself has not been declared GRAS, and
under US federal law is illegal to sell as a food, dietary supplement,
or animal feed.
State laws vary considerably as non-medical cannabis and derived
products have been legalized in some jurisdictions in the 2010s.
Similar to energy drinks and protein bars
which may contain vitamin or herbal additives, food and beverage items
can be infused with CBD as an alternative means of ingesting the
substance. In the United States, numerous products are marketed as containing CBD, but in reality contain little or none. Some companies marketing CBD-infused food products with claims that are similar to the effects of prescription drugs have received warning letters from the Food and Drug Administration for making unsubstantiated health claims. In February 2019, the New York City Department of Health announced plans to fine restaurants that sell food or drinks containing CBD, beginning in October 2019.
Sports
Cannabidiol has been used by professional and amateur athletes across disciplines and countries, with the World Anti-Doping Agency removing CBD from its banned substances list. The United States Anti-Doping Agency
and United Kingdom-Anti-Doping Agency do not have anti-CBD policies,
with the latter stating that, "CBD is not currently listed on the World
Anti-Doping Agency Prohibited List. As a result, it is permitted to use
in sport, though the intended benefits are unclear and not backed by
clinical evidence.. All other cannabinoids (including but not limited to
cannabis, hashish, marijuana, and THC) are prohibited in-competition.
The intention of the regulations is to prohibit cannabinoids that
activate the same receptors in the brain as activated by THC." In 2019, the cannabis manufacturer, Canopy Growth, acquired majority ownership of BioSteel Sports Nutrition, which is developing CBD products under endorsement by numerous professional athletes. The National Hockey League
Alumni Association began a project with Canopy Growth to determine if
CBD or other cannabis products might improve neurological symptoms and
quality of life in head-injured players. Numerous professional athletes use CBD, primarily for treating pain.
Legal status across countries
Australia
Prescription
medicine (Schedule 4) for therapeutic use containing two percent (2.0%)
or less of other cannabinoids commonly found in cannabis (such as ∆9-THC). A Schedule 4 drug under the SUSMP
is a Prescription Only Medicine, or Prescription Animal Remedy –
Substances, the use or supply of which should be by or on the order of
persons permitted by State or Territory legislation to prescribe and
should be available from a pharmacist on prescription.
In June 2020, the Australian Therapeutic Goods Administration
(TGA) published a consultation on a proposal to pave the way to make
"low dose" CBD available to consumer/patients via pharmacists only
through moving products from Schedule 4 to 3.
Any products sold would need to have their safety, quality and efficacy
pre-assessed by the TGA and be formally approved for sale (details to
be outlined by TGA). They would be made available to over 18s only, with
the maximum daily dose of 60 mg/day, up to 2% THC finished product
allowed, 30-day maximum supply, plant-derived or synthetic. This
proposal is based on an initial literature review on the safety of low
dose CBD published by the TGA in April 2020. Epidyolex was approved, for the adjunctive therapy of seizures associated with Lennox-Gastaut syndrome or with Dravet syndrome, on 18 September 2020, and added to the ARTG on 21 September 2020.
Bulgaria
In
2020, Bulgaria became the first country in the European Union to allow
retail sales of food products and supplements containing CBD, despite
the ongoing discussion within the EU about the classification of CBD as a
novel food.
But there exists a legal gap because of the lack of a
legally-permissible minimum amount of THC in the products containing
cannabinoids.
Canada
In October 2018, cannabidiol became legal for recreational and medical use by the federal Cannabis Act.
As of August 2019, CBD products in Canada could only be sold by
authorized retailers or federally licensed medical companies, limiting
their access to the general public.
The Canadian government states that CBD products "are subject to all of
the rules and requirements that apply to cannabis under the Cannabis
Act and its regulations."
It requires "a processing licence to manufacture products containing
CBD for sale, no matter what the source of the CBD is, and that CBD and
products containing CBD, such as cannabis oil, may only be sold by an
authorized retailer or licensed seller of medical CBD." Edible CBD products were scheduled to be permitted for sale in Canada on October 17, 2019 for human consumption.
As of August 2020, it is still illegal to carry cannabis and
cannabis-derived products (including products containing CBD) across the
Canadian border. If one carries any amount of cannabis for any purpose
(including medical), it need to be declared to the Canada Border Services Agency. Not declaring it is a serious criminal offence.
European Union
In 2019, the European Commission announced that CBD and other cannabinoids would be classified as "novel foods", meaning that CBD products would require authorization under the EU Novel Food Regulation
stating that because "this product was not used as a food or food
ingredient before May 15, 1997, before it may be placed on the market in
the EU as a food or food ingredient, a safety assessment under the
Novel Food Regulation is required."
The recommendation – applying to CBD extracts, synthesized CBD, and all
CBD products, including CBD oil – was scheduled for a final ruling by
the European Commission in March 2019.
If approved, manufacturers of CBD products would be required to conduct
safety tests and prove safe consumption, indicating that CBD products
would not be eligible for legal commerce until at least 2021. In December 2020, the European Commission concluded that CBD should not be considered as drug and can be qualified as food.
Cannabidiol is listed in the EU Cosmetics Ingredient Database (CosIng). However, the listing of an ingredient, assigned with an INCI name, in CosIng does not mean it is to be used in cosmetic products or is approved for such use.
Several industrial hemp varieties can be legally cultivated in Western Europe. A variety such as "Fedora 17" has a cannabinoid profile consistently around 1%, with THC less than 0.3%.
New Zealand
In
2017, the government made changes to the regulations so that
restrictions would be removed, which meant a doctor was able to
prescribe cannabidiol to patients.
The passing of the Misuse of Drugs (Medicinal Cannabis) Amendment Act
in December 2018 means cannabidiol is no longer a controlled drug in
New Zealand, but is a prescription medicine under the Medicines Act,
with the restriction that "the tetrahydrocannabinols (THCs) and
specified substances within the product must not exceed 2 percent of the
total CBD, tetrahydrocannabinol (THC) and other specified substances."
Russian Federation
According
to a document received in response to an appeal to the Ministry of
Internal Affairs of the Russian Federation, measures of state control in
the Russian Federation regarding CBD have not been established.
However, there is also a response from the Ministry of Health of the
Russian Federation indicating that CBD can be considered as an isomer of
restricted THC. But in this case, the THC isomer is also, for example, progesterone, which is freely sold in pharmacies. On February 17, 2020, the deputy of the Moscow City Duma Darya Besedina
sent an official request to the Prime Minister of the Russian
Federation Mikhail Mishustin with a request to eliminate that legal
ambiguity by publishing official explanations and, if necessary, making
required changes in the corresponding government decree.
Sweden
Cannabidiol is classified as a medical product in Sweden. However, in July 2019, Supreme Court of Sweden ruled that CBD oil with any concentration of THC falls under the narcotic control laws.
Switzerland
While
THC remains illegal, cannabidiol is not subject to the Swiss Narcotic
Acts because this substance does not produce a comparable psychoactive
effect. Cannabis products containing less than 1% THC can be sold and purchased legally.
Ukraine
On 7 April 2021 the Ukrainian government legalised use of isolated cannabidiol. Additionally, it approved Nabiximols, a cannabidiol-containing drug, for medical use.
United Kingdom
Cannabidiol, in an oral-mucosal spray formulation combined with Delta-9-tetrahydrocannabinol,
is a product available (by prescription only until 2017) for the relief
of severe spasticity due to multiple sclerosis (where other anti-spasmodics have not been effective).
Until 2017, products containing cannabidiol marketed for medical purposes were classed as medicines by the UK regulatory body, the Medicines and Healthcare products Regulatory Agency (MHRA) and could not be marketed without regulatory approval for the medical claims. As of 2018,
cannabis oil is legal to possess, buy, and sell in the UK, providing
the product does not contain more than 1 milligram of THC and is not
advertised as providing a medicinal benefit.
In January 2019, the UK Food Standards Agency indicated it would regard CBD products, including CBD oil, as a novel food
having no history of use before May 1997, and stated that such products
must have authorisation and proven safety before being marketed.
The deadline for companies with existing products to submit a full and
validated novel foods application with the FSA is 31 March 2021; failure
to do so before this date will exclude those companies from selling
CBD. New products containing CBD after this deadline will require a fully approved application.
United Nations
Cannabidiol is not scheduled under the Convention on Psychotropic Substances or any other UN drug treaties. In 2018, the World Health Organization recommended that CBD remain unscheduled.
United States
As of October 2020, cannabidiol extracted from marijuana remains a Schedule I Controlled Substance, and is not approved as a prescription drug or dietary supplement or allowed for interstate commerce in the United States.
CBD derived from hemp (with 0.3% THC or lower) is legal to sell as a
cosmetics ingredient, but cannot be sold under federal law as an
ingredient in food, dietary supplement, or animal food. It is a common misconception that the legal ability to sell hemp (which may contain CBD) makes CBD legal.
In September 2018, following its approval by the FDA for rare types of childhood epilepsy, Epidiolex was rescheduled (by the Drug Enforcement Administration) as a Schedule V drug to allow for its prescription use.[149] This allows GW Pharmaceuticals to sell Epidiolex, but it does not apply broadly and all other CBD-containing products remain Schedule I drugs. Epidiolex still requires rescheduling in some states before it can be prescribed in those states.
In 2013, a CNN program that featured Charlotte's Web cannabis brought increased attention to the use of CBD in the treatment of seizure disorders.
Since then, 16 states have passed laws to allow the use of CBD
products with a physician's recommendation (instead of a prescription)
for treatment of certain medical conditions. This is in addition to the 30 states
that have passed comprehensive medical cannabis laws, which allow for
the use of cannabis products with no restrictions on THC content.
Of these 30 states, eight have legalized the use and sale of cannabis
products without requirement for a physician's recommendation. As of October 2020, CBD was not an FDA-approved drug eligible for interstate commerce, and the FDA encouraged manufacturers to follow procedures for drug approval.
Some manufacturers ship cannabidiol products nationally, an
illegal action which the FDA did not enforce in 2018, with CBD remaining
the subject of an FDA investigational new drug evaluation, and is not considered legal as a dietary supplement or food ingredient, as of October 2020. Federal illegality has made it difficult historically to conduct research on CBD. CBD is openly sold in head shops and health food stores in some states where such sales have not been explicitly legalized.
State and local governments may also regulate cannabidiol. For
example, the Massachusetts Department of Agricultural Resources issued a
rule in June 2019 aligning state CBD regulations with FDA regulations.
This means that although recreational marijuana is legal in the state,
CBD cannot legally be sold in food or as a dietary supplement under
state law.
Health concerns
In November 2019, the FDA issued concerns about the safety of cannabidiol, stating that CBD use has potential to cause liver injury, interfere with the mechanisms of prescription drugs, produce gastrointestinal disorders, or affect alertness and mood. In October 2020, the FDA updated its safety concerns about CBD,
acknowledging the unknown effects of protracted use, how it affects the
developing brain, fetus or infants during breastfeeding, whether it
interacts with dietary supplements or prescription drugs, whether male fertility is affected, and its possible side effects, such as drowsiness.
In February 2020, the UK FSA advised vulnerable people, such as
pregnant women, breastfeeding mothers, and those already taking
medication for other medical concerns not to take CBD. The FSA further
recommended that healthy adults should not consume more than 70 mg CBD
per day.
Mislabeling and poisoning
Studies
conducted by the FDA from 2014 through 2019 have determined that a
majority of CBD products are not accurately labeled with the amount of
CBD they contain. For example, a 2017 analysis of cannabidiol content in oil, tincture, or liquid vape
products purchased online in the United States showed that 69% were
mislabeled, with 43% having higher and 26% having lower content than
stated on product labels.
In 2020, the FDA conducted a study of 147 CBD products and found that half contained THC.
As of September 2019, 1,085 people contacted US poison control centers
about CBD-induced illnesses, doubling the number of cases over the 2018
rate and increasing by 9 times the case numbers of 2017. Of cases reported in 2019, more than 33% received medical attention and 46 people were admitted to a hospital intensive care unit, possibly due to exposure to other products, or drug interactions with CBD.
2018 Farm Bill and hemp
The 2014 Farm Bill legalized the sale of "non-viable hemp material" grown within states participating in the Hemp Pilot Program which defined hemp as cannabis containing less than 0.3% of THC. Although the 2018 United States Farm Bill
led some states to interpret the bill as enabling private farmers to
grow hemp for extraction and retail of CBD, federal agencies – including
the FDA and DEA – retained regulatory authority over hemp-derived CBD as a Schedule I substance.
By federal law, private enterprises developing hemp-derived CBD are
obligated to cultivate hemp exclusively for industrial purposes, which
involve the fiber and seed, but not the flowering tops which contain THC
and CBD. Hemp CBD products may not be sold into general commerce, but rather are allowed only for research.
The 2018 Farm Bill requires that research and development of CBD for a
therapeutic purpose would have to be conducted under notification and
reporting to the FDA.
FDA warning letters
From 2015 to December 2020, the FDA issued dozens of warning letters to American manufacturers of CBD products for false advertising and illegal interstate marketing of CBD as an unapproved drug to treat diseases, such as cancer, osteoarthritis, symptoms of opioid withdrawal, Alzheimer's disease, and pet disorders.
The FDA said that the letters were issued to enforce action against
companies that were deceiving consumers by marketing illegal products
for which there was insufficient evidence of safety and efficacy to
treat diseases.
In July 2019, the FDA stated: "Selling unapproved products with
unsubstantiated therapeutic claims — such as claims that CBD products
can treat serious diseases and conditions — can put patients and
consumers at risk by leading them to put off important medical care.
Additionally, there are many unanswered questions about the science,
safety, effectiveness and quality of unapproved products containing
CBD."
In December 2020, the Federal Trade Commission (FTC) initiated a law enforcement crackdown on American companies marketing CBD products as unapproved drugs. The warning also applied to hemp CBD capsules and oil that were being marketed illegally while not adhering to the federal definition of a dietary supplement.