From Wikipedia, the free encyclopedia
| Xenotransplantation |
|---|
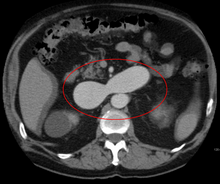
Long
axis echocardiography. Representative long axis view echocardiography, 4
weeks after myocardial infarction (MI), right before CMPC/placebo
infusion. Thinning and akinesia of the septal apical wall due to MI can
be appreciated. |
| MeSH | D014183 |
|---|
|
Xenotransplantation (xenos- from the Greek meaning "foreign" or strange), or heterologous transplant, is the transplantation of living cells, tissues or organs from one species to another. Such cells, tissues or organs are called xenografts or xenotransplants. It is contrasted with allotransplantation (from other individual of same species), syngeneic
transplantation or isotransplantation (grafts transplanted between two
genetically identical individuals of the same species) and autotransplantation (from one part of the body to another in the same person).
Xenotransplantation of human tumor cells into immunocompromised mice is a research technique frequently used in pre-clinical oncology research.
Human xenotransplantation offers a potential treatment for end-stage organ failure, a significant health problem in parts of the industrialized world. It also raises many novel medical, legal and ethical issues. A continuing concern is that many animals, such as pigs, have a shorter lifespan than humans, meaning that their tissues age at a quicker rate. Disease transmission (xenozoonosis) and permanent alteration to the genetic code of animals are also causes for concern. Similarly to objections to animal testing, animal rights activists have also objected to xenotransplantation on ethical grounds. A few temporarily successful cases of xenotransplantation are published.
It is common for patients and physicians to use the term
"allograft" imprecisely to refer to either allograft (human-to-human) or
xenograft (animal-to-human), but it is helpful scientifically (for
those searching or reading the scientific literature) to maintain the more precise distinction in usage.
History
The
first serious attempts at xenotransplantation (then called
heterotransplantation) appeared in the scientific literature in 1905,
when slices of rabbit kidney were transplanted into a child with chronic kidney disease.
In the first two decades of the 20th century, several subsequent
efforts to use organs from lambs, pigs, and primates were published.
Scientific interest in xenotransplantation declined when the immunological basis of the organ rejection process was described. The next waves of studies on the topic came with the discovery of immunosuppressive drugs. Even more studies followed Dr. Joseph Murray's first successful renal transplantation
in 1954 and scientists, facing the ethical questions of organ donation
for the first time, accelerated their effort in looking for alternatives
to human organs.
In 1963, doctors at Tulane University attempted chimpanzee-to-human
renal transplantations in six people who were near death; after this
and several subsequent unsuccessful attempts to use primates as organ
donors and the development of a working cadaver organ procuring program,
interest in xenotransplantation for kidney failure dissipated.
Out of 13 such transplants performed by Keith Reemtsma, one kidney
recipient lived for 9 months, returning to work as a schoolteacher. At
autopsy, the chimpanzee kidneys appeared normal and showed no signs of
acute or chronic rejection.
An American infant girl known as "Baby Fae" with hypoplastic left heart syndrome was the first infant recipient of a xenotransplantation, when she received a baboon heart in 1984. The procedure was performed by Leonard Lee Bailey at Loma Linda University Medical Center in Loma Linda, California. Fae died 21 days later due to a humoral-based graft rejection thought to be caused mainly by an ABO blood type
mismatch, considered unavoidable due to the rarity of type O baboons.
The graft was meant to be temporary, but unfortunately a suitable allograft
replacement could not be found in time. While the procedure itself did
not advance the progress on xenotransplantation, it did shed a light on
the insufficient amount of organs for infants. The story grew so big
that it made such an impact that the crisis of infant organ shortage
improved for that time.
Xenotransplantation of human tumor cells into immunocompromised
mice is a research technique frequently used in oncology research.
It is used to predict the sensitivity of the transplanted tumor to
various cancer treatments; several companies offer this service,
including the Jackson Laboratory.
Human organs have been transplanted into animals as a powerful research technique for studying human biology
without harming human patients. This technique has also been proposed
as an alternative source of human organs for future transplantation into
human patients. For example, researchers from the Ganogen Research Institute transplanted human fetal kidneys into rats which demonstrated life supporting function and growth.
Potential uses
A
worldwide shortage of organs for clinical implantation causes about
20–35% of patients who need replacement organs to die on the waiting
list.
Certain procedures, some of which are being investigated in early
clinical trials, aim to use cells or tissues from other species to treat
life-threatening and debilitating illnesses such as cancer, diabetes, liver failure and Parkinson's disease. If vitrification
can be perfected, it could allow for long-term storage of xenogenic
cells, tissues and organs so that they would be more readily available
for transplant.
Xenotransplants could save thousands of patients waiting for
donated organs. The animal organ, probably from a pig or baboon could be
genetically altered with human genes to trick a patient's immune system into accepting it as a part of its own body. They have re-emerged because of the lack of organs available and the constant battle to keep immune systems from rejecting allotransplants. Xenotransplants are thus potentially a more effective alternative.
Xenotransplantation also is and has been a valuable tool used in research laboratories to study developmental biology.
Patient derived tumor xenografts in animals can be used to test treatments.
Potential animal organ donors
Since
they are the closest relatives to humans, non-human primates were first
considered as a potential organ source for xenotransplantation to
humans. Chimpanzees were originally considered the best option since
their organs are of similar size, and they have good blood type
compatibility with humans, which makes them potential candidates for xenotransfusions.
However, since chimpanzees are listed as an endangered species, other
potential donors were sought. Baboons are more readily available, but
impractical as potential donors. Problems include their smaller body
size, the infrequency of blood group O (the universal donor), their long
gestation period, and their typically small number of offspring. In
addition, a major problem with the use of nonhuman primates is the
increased risk of disease transmission, since they are so closely
related to humans.
Pigs (Sus scrofa domesticus)
are currently thought to be the best candidates for organ donation. The
risk of cross-species disease transmission is decreased because of
their increased phylogenetic distance from humans. Pigs have relatively short gestation periods, large litters, and are easy to breed making them readily available.
They are inexpensive and easy to maintain in pathogen-free facilities,
and current gene editing tools are adapted to pigs to combat rejection
and potential zoonoses.
Pig organs are anatomically comparable in size, and new infectious
agents are less likely since they have been in close contact with humans
through domestication for many generations.
Treatments sourced from pigs have proven to be successful such as porcine-derived insulin for patients with diabetes mellitus.
Increasingly, genetically engineered pigs are becoming the norm, which
raises moral qualms, but also increases the success rate of the
transplant.
Current experiments in xenotransplantation most often use pigs as the donor, and baboons as human models.
In the field of regenerative medicine, pancreatogenesis- or
nephrogenesis-disabled pig embryos, unable to form a specific organ,
allow experimentation toward the in vivo generation of functional
organs from xenogenic pluripotent stem cells in large animals via
compensation for an empty developmental niche (blastocyst
complementation).
Such experiments provide the basis for potential future application of
blastocyst complementation to generate transplantable human organs from
the patient's own cells, using livestock animals, to increase quality
of life for those with end-stage organ failure.
Barriers and issues
Immunologic barriers
To
date, no xenotransplantation trials have been entirely successful due
to the many obstacles arising from the response of the recipient's immune system.
"Xenozoonoses" are one of the biggest threats to rejections, as they
are xenogenetic infections. The introduction of these microorganisms are
a big issue that lead to the fatal infections and then rejection of the
organs.
This response, which is generally more extreme than in
allotransplantations, ultimately results in rejection of the xenograft,
and can in some cases result in the immediate death of the recipient.
There are several types of rejection organ xenografts are faced with,
these include hyperacute rejection, acute vascular rejection, cellular
rejection, and chronic rejection.
A rapid, violent, and hyperacute response comes as a result of antibodies present in the host organism. These antibodies are known as xenoreactive natural antibodies (XNAs).
Hyperacute rejection
This
rapid and violent type of rejection occurs within minutes to hours from
the time of the transplant. It is mediated by the binding of XNAs
(xenoreactive natural antibodies) to the donor endothelium, causing
activation of the human complement system,
which results in endothelial damage, inflammation, thrombosis and
necrosis of the transplant. XNAs are first produced and begin
circulating in the blood in neonates, after colonization of the bowel by
bacteria with galactose moieties on their cell walls. Most of these
antibodies are the IgM class, but also include IgG, and IgA.
The epitope XNAs target is an α-linked galactose moiety,
Gal-α-1,3Gal (also called the α-Gal epitope), produced by the enzyme
α-galactosyl transferase. Most non-primates contain this enzyme thus, this epitope is present on the organ epithelium and is perceived as a foreign antigen
by primates, which lack the galactosyl transferase enzyme. In pig to
primate xenotransplantation, XNAs recognize porcine glycoproteins of the
integrin family.
The binding of XNAs initiate complement activation through the classical complement pathway.
Complement activation causes a cascade of events leading to:
destruction of endothelial cells, platelet degranulation, inflammation,
coagulation, fibrin deposition, and hemorrhage. The end result is thrombosis and necrosis of the xenograft.
Overcoming hyperacute rejection
Since
hyperacute rejection presents such a barrier to the success of
xenografts, several strategies to overcome it are under investigation:
Interruption of the complement cascade
- The recipient's complement cascade can be inhibited through the
use of cobra venom factor (which depletes C3), soluble complement
receptor type 1, anti-C5 antibodies, or C1 inhibitor (C1-INH).
Disadvantages of this approach include the toxicity of cobra venom
factor, and most importantly these treatments would deprive the
individual of a functional complement system.
Transgenic organs (Genetically engineered pigs)
- 1,3 galactosyl transferase gene knockouts – These pigs don't
contain the gene that codes for the enzyme responsible for expression of
the immunogeneic gal-α-1,3Gal moiety (the α-Gal epitope).
- Increased expression of H-transferase (α 1,2 fucosyltransferase), an
enzyme that competes with galactosyl transferase. Experiments have
shown this reduces α-Gal expression by 70%.
- Expression of human complement regulators (CD55, CD46, and CD59) to inhibit the complement cascade.
- Plasmaphoresis, on humans to remove 1,3 galactosyltransferase,
reduces the risk of activation of effector cells such as CTL (CD8 T
cells), complement pathway activation and delayed type hypersensitivity
(DTH).
Acute vascular rejection
Also
known as delayed xenoactive rejection, this type of rejection occurs in
discordant xenografts within 2 to 3 days, if hyperacute rejection is
prevented. The process is much more complex than hyperacute rejection
and is currently not completely understood. Acute vascular rejection
requires de novo protein synthesis and is driven by interactions between
the graft endothelial cells and host antibodies, macrophages, and
platelets. The response is characterized by an inflammatory infiltrate
of mostly macrophages and natural killer cells (with small numbers of T cells), intravascular thrombosis, and fibrinoid necrosis of vessel walls.
Binding of the previously mentioned XNAs to the donor endothelium
leads to the activation of host macrophages as well as the endothelium
itself. The endothelium activation is considered type II since gene
induction and protein synthesis are involved. The binding of XNAs
ultimately leads to the development of a procoagulant state, the
secretion of inflammatory cytokines and chemokines, as well as expression of leukocyte adhesion molecules such as E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1).
This response is further perpetuated as normally binding between
regulatory proteins and their ligands aid in the control of coagulation
and inflammatory responses. However, due to molecular incompatibilities
between the molecules of the donor species and recipient (such as
porcine major histocompatibility complex molecules and human natural killer cells), this may not occur.
Overcoming acute vascular rejection
Due
to its complexity, the use of immunosuppressive drugs along with a
wide array of approaches are necessary to prevent acute vascular
rejection, and include administering a synthetic thrombin inhibitor to
modulate thrombogenesis, depletion of anti-galactose antibodies (XNAs)
by techniques such as immunoadsorption, to prevent endothelial cell
activation, and inhibiting activation of macrophages (stimulated by CD4+
T cells) and NK cells (stimulated by the release of Il-2). Thus, the
role of MHC molecules and T cell responses in activation would have to
be reassessed for each species combo.
Accommodation
If
hyperacute and acute vascular rejection are avoided accommodation is
possible, which is the survival of the xenograft despite the presence of
circulating XNAs. The graft is given a break from humoral rejection
when the complement cascade is interrupted, circulating antibodies are
removed, or their function is changed, or there is a change in the
expression of surface antigens on the graft. This allows the xenograft
to up-regulate and express protective genes, which aid in resistance to
injury, such as heme oxygenase-1 (an enzyme that catalyzes the degradation of heme).
Cellular rejection
Rejection of the xenograft in hyperacute and acute vascular rejection is due to the response of the humoral immune system, since the response is elicited by the XNAs. Cellular rejection is based on cellular immunity,
and is mediated by natural killer cells that accumulate in and damage
the xenograft and T-lymphocytes which are activated by MHC molecules
through both direct and indirect xenorecognition.
In direct xenorecognition, antigen presenting cells from the xenograft present peptides to recipient CD4+ T cells via xenogeneic MHC class II molecules, resulting in the production of interleukin 2
(IL-2). Indirect xenorecognition involves the presentation of antigens
from the xenograft by recipient antigen presenting cells to CD4+ T cells. Antigens of phagocytosed graft cells can also be presented by the host's class I MHC molecules to CD8+ T cells.
The strength of cellular rejection in xenografts remains
uncertain, however, it is expected to be stronger than in allografts due
to differences in peptides among different animals. This leads to more
antigens potentially recognized as foreign, thus eliciting a greater
indirect xenogenic response.
Overcoming cellular rejection
A proposed strategy to avoid cellular rejection is to induce donor non-responsiveness using hematopoietic chimerism.
Donor stem cells are introduced into the bone marrow of the recipient,
where they coexist with the recipient's stem cells. The bone marrow stem
cells give rise to cells of all hematopoietic lineages, through the
process of hematopoiesis.
Lymphoid progenitor cells are created by this process and move to the
thymus where negative selection eliminates T cells found to be reactive
to self. The existence of donor stem cells in the recipient's bone
marrow causes donor reactive T cells to be considered self and undergo apoptosis.
Chronic rejection
Chronic rejection is slow and progressive, and usually occurs in transplants that survive the initial rejection phases.
Scientists are still unclear how chronic rejection exactly works,
research in this area is difficult since xenografts rarely survive past
the initial acute rejection phases. Nonetheless, it is known that XNAs
and the complement system are not primarily involved. Fibrosis
in the xenograft occurs as a result of immune reactions, cytokines
(which stimulate fibroblasts), or healing (following cellular necrosis
in acute rejection). Perhaps the major cause of chronic rejection is arteriosclerosis. Lymphocytes, which were previously activated by antigens in the vessel
wall of the graft, activate macrophages to secrete smooth muscle growth
factors. This results in a build up of smooth muscle cells on the vessel
walls, causing the hardening and narrowing of vessels within the graft.
Chronic rejection leads to pathologic changes of the organ, and is why
transplants must be replaced after so many years. It is also anticipated that chronic rejection will be more aggressive in xenotransplants as opposed to allotransplants.
Dysregulated coagulation
Successful
efforts have been made to create knockout mice without α1,3GT; the
resulting reduction in the highly immunogenic αGal epitope has resulted
in the reduction of the occurrence of hyperacute rejection, but has not
eliminated other barriers to xenotransplantation such as dysregulated
coagulation, also known as coagulopathy.
Different organ xenotransplants result in different responses in
clotting. For example, kidney transplants result in a higher degree of coagulopathy, or impaired clotting, than cardiac transplants, whereas liver xenografts result in severe thrombocytopenia, causing recipient death within a few days due to bleeding. An alternate clotting disorder, thrombosis,
may be initiated by preexisting antibodies that affect the protein C
anticoagulant system. Due to this effect, porcine donors must be
extensively screened before transplantation. Studies have also shown
that some porcine transplant cells are able to induce human tissue
factor expression, thus stimulating platelet and monocyte aggregation
around the xenotransplanted organ, causing severe clotting. Additionally, spontaneous platelet accumulation may be caused by contact with pig von Willebrand factor.
Just as the α1,3G epitope is a major problem in
xenotransplantation, so too is dysregulated coagulation a cause of
concern. Transgenic pigs that can control for variable coagulant
activity based on the specific organ transplanted would make
xenotransplantation a more readily available solution for the 70,000
patients per year who do not receive a human donation of the organ or
tissue they need.
Physiology
Extensive
research is required to determine whether animal organs can replace the
physiological functions of human organs. Many issues include size –
differences in organ size limit the range of potential recipients of
xenotransplants; longevity – The lifespan of most pigs is roughly 15
years, currently it is unknown whether or not a xenograft may be able to
last longer than that; hormone and protein differences – some proteins
will be molecularly incompatible, which could cause malfunction of
important regulatory processes. These differences also make the prospect
of hepatic xenotransplantation less promising, since the liver plays an
important role in the production of so many proteins;
environment – for example, pig hearts work in a different anatomical
site and under different hydrostatic pressure than in humans;
temperature – the body temperature of pigs is 39 °C (2 °C above the
average human body temperature). Implications of this difference, if
any, on the activity of important enzymes are currently unknown.
Xenozoonosis
Xenozoonosis, also known as zoonosis
or xenosis, is the transmission of infectious agents between species
via xenograft. Animal to human infection is normally rare, but has
occurred in the past. An example of such is the avian influenza, when an influenza A virus was passed from birds to humans.
Xenotransplantation may increase the chance of disease transmission for
3 reasons: (1) implantation breaches the physical barrier that normally
helps to prevent disease transmission, (2) the recipient of the
transplant will be severely immunosuppressed, and (3) human complement
regulators (CD46, CD55, and CD59) expressed in transgenic pigs have been
shown to serve as virus receptors, and may also help to protect viruses
from attack by the complement system.
Examples of viruses carried by pigs include porcine herpesvirus, rotavirus, parvovirus, and circovirus.
Porcine herpesviruses and rotaviruses can be eliminated from the donor
pool by screening, however others (such as parvovirus and circovirus)
may contaminate food and footwear then re-infect the herd. Thus, pigs to
be used as organ donors must be housed under strict regulations and
screened regularly for microbes and pathogens. Unknown viruses, as well
as those not harmful in the animal, may also pose risks.
Of particular concern are PERVS (porcine endogenous retroviruses),
vertically transmitted microbes that embed in swine genomes. The risks
with xenosis are twofold, as not only could the individual become
infected, but a novel infection could initiate an epidemic in the human
population. Because of this risk, the FDA has suggested any recipients
of xenotransplants shall be closely monitored for the remainder of their
life, and quarantined if they show signs of xenosis.
Baboons and pigs carry myriad transmittable agents that are
harmless in their natural host, but extremely toxic and deadly in
humans. HIV is an example of a disease believed to have jumped from
monkeys to humans. Researchers also do not know if an outbreak of
infectious diseases could occur and if they could contain the outbreak
even though they have measures for control. Another obstacle facing
xenotransplants is that of the body's rejection of foreign objects by
its immune system. These antigens (foreign objects) are often treated
with powerful immunosuppressive drugs that could, in turn, make the
patient vulnerable to other infections and actually aid the disease.
This is the reason the organs would have to be altered to fit the
patients' DNA (histocompatibility).
In 2005, the Australian National Health and Medical Research Council
(NHMRC) declared an eighteen-year moratorium on all animal-to-human
transplantation, concluding that the risks of transmission of animal
viruses to patients and the wider community had not been resolved.
This was repealed in 2009 after an NHMRC review stated "... the risks,
if appropriately regulated, are minimal and acceptable given the
potential benefits.", citing international developments on the
management and regulation of xenotransplantation by the World Health
Organisation and the European Medicines Agency.
Porcine endogenous retroviruses
Endogenous retroviruses
are remnants of ancient viral infections, found in the genomes of most,
if not all, mammalian species. Integrated into the chromosomal DNA,
they are vertically transferred through inheritance.
Due to the many deletions and mutations they accumulate over time, they
usually are not infectious in the host species, however the virus may
become infectious in another species. PERVS were originally discovered as retrovirus particles released from cultured porcine kidney cells. Most breeds of swine harbor approximately 50 PERV genomes in their DNA.
Although it is likely that most of these are defective, some may be
able to produce infectious viruses so every proviral genome must be
sequenced to identify which ones pose a threat. In addition, through
complementation and genetic recombination, two defective PERV genomes
could give rise to an infectious virus.
There are three subgroups of infectious PERVs (PERV-A, PERV-B, and
PERV-C). Experiments have shown that PERV-A and PERV-B can infect human
cells in culture.
To date no experimental xenotransplantations have demonstrated PERV
transmission, yet this does not mean PERV infections in humans are
impossible. Pig cells have been engineered to inactivate all 62 PERVs in the genome using CRISPR Cas9 genome editing technology, and eliminated infection from the pig to human cells in culture.
Ethics
Xenografts
have been a controversial procedure since they were first attempted.
Many, including animal rights groups, strongly oppose killing animals to
harvest their organs for human use.
In the 1960s, many organs came from the chimpanzees, and were
transferred into people that were deathly ill, and in turn, did not live
much longer afterwards.
Modern scientific supporters of xenotransplantation argue that the
potential benefits to society outweigh the risks, making pursuing
xenotransplantation the moral choice. None of the major religions object to the use of genetically modified pig organs for life-saving transplantation. Religions such as Buddhism and Jainism, however, have long espoused non-violence towards all living creatures.
In general, the use of pig and cow tissue in humans has been met with
little resistance, save some religious beliefs and a few philosophical
objections. Experimentation without consent doctrines are now followed,
which was not the case in the past, which may lead to new religious
guidelines to further medical research on pronounced ecumenical
guidelines. The "Common Rule" is the United States bio-ethics mandate as
of 2011.
History of Xenotransplantation in Ethics
At
the beginning of the 20th century when studies in Xenotransplantation
were just beginning, few questioned the morality of it, turning to
animals as a "natural" alternative to allografts. While satirical plays mocked Xenografters such as Serge Voronoff,
and some images showing emotionally distraught primates appeared - who
Voronoff had deprived of their testicles - no serious attempts were yet
made to question the science based on animal rights concerns. Xenotransplantation was not taken seriously, at least in France, during the first half of the 20th century.
With the Baby Fae incident of 1984 as the impetus, animal rights
activists began to protest, gathering media attention and proving that
some people felt that it was unethical and a violation of the animal's
own rights to use its organs to preserve a sick human's life. Treating animals as mere tools for the slaughter on demand by human will would lead to a world they would not prefer. Supporters of the transplant pushed back, claiming that saving a human life justifies the sacrifice of an animal one. Most animal rights activists found the use of primate organs more reprehensible than those of, for example, pigs.
As Peter Singer et al. have expressed, many primates exhibit greater
social structure, communication skills, and affection than mentally
deficient humans and human infants.
Despite this, it is considerably unlikely that animal suffering will
provide sufficient impetus for regulators to prevent
xenotransplantation.
Informed consent of patient
Autonomy and informed consent
are important when considering the future uses of xenotransplantation. A
patient undergoing xenotransplantation should be fully aware of the
procedure and should have no outside force influencing their choice.
The patient should understand the risks and benefits of such a
transplantation. However, it has been suggested that friends and family
members should also give consent, because the repercussions of
transplantation are high, with the potential of diseases and viruses
crossing over to humans from the transplantation. Close contacts are at
risk for such infections. Monitoring of close relations may also be
required to ensure that xenozoonosis
is not occurring. The question then becomes: does the autonomy of the
patient become limited based on the willingness or unwillingness of
friends and family to give consent, and are the principles of confidentiality broken?
The safety of public health is a factor to be considered. If there is any risk to the public at all for an outbreak
from transplantation there must be procedures in place to protect the
public. Not only does the recipient of the transplantation have to
understand the risks and benefits, but society must also understand and
consent to such an agreement.
The Ethics Committee of the International Xenotransplantation
Association points out one major ethical issue is the societal response
to such a procedure.
The assumption is that the recipient of the transplantation will be
asked to undergo lifelong monitoring, which would deny the recipient the
ability to terminate the monitoring at any time, which is in direct
opposition of the Declaration of Helsinki and the US Code of Federal Regulations.
In 2007, xenotransplantation was banned under ethical grounds in all
countries but Argentina, Russia and New Zealand. Since then, the
practice has only been carried out to treatment for diabetes type 1 to
serve as a substitute for insulin injections.
The application of the four bioethics principal is found to be
everywhere because it is now standardized in the moral conducts of a
laboratory.
The four principles emphasize on the informed consent, the Hippocratic
Oath to do no harm, apply one's skill to help others, and protecting the
rights of others to quality care.
Problems with xenotransplantation is that even though it has
future medical benefits, it also has the serious risk of introducing and
spreading the infectious diseases, into the human population.
There have been guidelines that have been drafted by the government
that have the purpose of forming the foundation of infectious disease
surveillance. In the United Kingdom,
the guideline that were introduced state that first, "the periodic
provision of bodily samples that would then be archived for
epidemiological purposes;" second, "post-mortem analysis in case of
death, the storage of samples post-mortem, and the disclosure of this
agreement to their family;" third, "refrain from donating blood, tissue
or organs;" fourth, "the use of barrier contraception when engaging in
sexual intercourse;" fifth, keep both name and current address on
register and to notify the relevant health authorities when moving
abroad;" and lastly "divulge confidential information, including one's
status as a xenotransplantation recipient to researchers, all health
care professionals from whom one seeks professional services, and close
contacts such as current and future sexual partners."
With these guidelines in place the patient has to abide to these rules
until either their lifetime or until the government determines that
there is no need for public health safe guards.
Xenotransplantation guidelines in the United States
The Food and Drug Administration (FDA)
has also stated that if a transplantation takes place the recipient
must undergo monitoring for the rest of that recipient's lifetime and
waive their right to withdraw. The reason for requiring lifelong
monitoring is due to the risk of acute infections that may occur. The
FDA suggests that a passive screening program should be implemented and
should extend for the life of the recipient.