From Wikipedia, the free encyclopedia
The
Clausius–Clapeyron relation, named after
Rudolf Clausius[1] and
Benoît Paul Émile Clapeyron,
[2] is a way of characterizing a discontinuous
phase transition between two
phases of matter of a single constituent. On a
pressure–
temperature (P–T) diagram, the line separating the two phases is known as the coexistence curve. The Clausius–Clapeyron relation gives the
slope of the
tangents to this curve. Mathematically,

where

is the slope of the tangent to the coexistence curve at any point,

is the specific
latent heat,

is the
temperature,

is the
specific volume change of the phase transition, and

is the
specific entropy change of the phase transition.
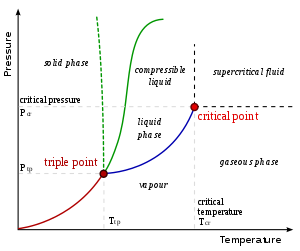
Derivations
A typical phase diagram. The dotted green line gives the anomalous
behavior of water. The Clausius–Clapeyron relation can be used to find
the relationship between pressure and temperature along
phase boundaries.
Derivation from state postulate
Using the
state postulate, take the
specific entropy 
for a homogeneous substance to be a function of
specific volume 
and
temperature 
.
[3]:508

The Clausius–Clapeyron relation characterizes behavior of a
closed system during a
phase change, during which temperature and
pressure are constant by definition. Therefore,
[3]:508

Using the appropriate
Maxwell relation gives
[3]:508

where

is the pressure. Since pressure and temperature are constant, by
definition the derivative of pressure with respect to temperature does
not change.
[4][5]:57, 62 & 671 Therefore, the
partial derivative of specific entropy may be changed into a
total derivative

and the total derivative of pressure with respect to temperature may be
factored out when
integrating from an initial phase

to a final phase

,
[3]:508 to obtain

where

and

are respectively the change in specific entropy and specific volume. Given that a phase change is an internally
reversible process, and that our system is closed, the
first law of thermodynamics holds

where

is the
internal energy of the system. Given constant pressure and temperature (during a phase change) and the definition of
specific enthalpy 
, we obtain



Given constant pressure and temperature (during a phase change), we obtain
[3]:508

Substituting the definition of
specific latent heat 
gives

Substituting this result into the pressure derivative given above (

), we obtain
[3]:508[6]

This result (also known as the
Clapeyron equation) equates the slope of the tangent to the
coexistence curve 
, at any given point on the curve, to the function

of the specific latent heat

, the temperature

, and the change in specific volume

.
Derivation from Gibbs–Duhem relation
Suppose two phases,

and

, are in contact and at equilibrium with each other. Their chemical potentials are related by

Furthermore, along the
coexistence curve,

One may therefore use the
Gibbs–Duhem relation

(where

is the specific
entropy,

is the
specific volume, and

is the
molar mass) to obtain

Rearrangement gives

from which the derivation of the Clapeyron equation continues as in
the previous section.
Ideal gas approximation at low temperatures
When the
phase transition of a substance is between a
gas phase and a condensed phase (
liquid or
solid), and occurs at temperatures much lower than the
critical temperature of that substance, the
specific volume of the gas phase

greatly exceeds that of the condensed phase

. Therefore, one may approximate

at low
temperatures. If
pressure is also low, the gas may be approximated by the
ideal gas law, so that

where

is the pressure,

is the
specific gas constant, and

is the temperature. Substituting into the Clapeyron equation

we can obtain the
Clausius–Clapeyron equation[3]:509

for low temperatures and pressures,
[3]:509 where

is the
specific latent heat of the substance.
Let

and

be any two points along the
coexistence curve between two phases

and

. In general,

varies between any two such points, as a function of temperature. But if

is constant,



or
[5]:672

These last equations are useful because they relate
equilibrium or
saturation vapor pressure and temperature to the latent heat of the phase change,
without requiring specific volume data.
Applications
Chemistry and chemical engineering
For transitions between a gas and a condensed phase with the approximations described above, the expression may be rewritten as

where

is a constant. For a liquid-gas transition,

is the
specific latent heat (or
specific enthalpy) of
vaporization; for a solid-gas transition,

is the specific latent heat of
sublimation. If the latent heat is known, then knowledge of one point on the
coexistence curve determines the rest of the curve. Conversely, the relationship between

and

is linear, and so
linear regression is used to estimate the latent heat.
Meteorology and climatology
Atmospheric water vapor drives many important
meteorologic phenomena (notably
precipitation), motivating interest in its
dynamics. The Clausius–Clapeyron equation for water vapor under typical atmospheric conditions (near
standard temperature and pressure) is

where:
The temperature dependence of the latent heat

, and therefore of the saturation vapor pressure

,
cannot be neglected in this application. Fortunately, the
August-Roche-Magnus formula provides a very good approximation, using pressure in
hPa and temperature in
Celsius:
 [7][8]
[7][8]
(This is also sometimes called the
Magnus or
Magnus-Tetens approximation, though this attribution is historically inaccurate.
[9])
Under typical atmospheric conditions, the
denominator of the
exponent depends weakly on

(for which the unit is Celsius). Therefore, the August-Roche-Magnus
equation implies that saturation water vapor pressure changes
approximately
exponentially
with temperature under typical atmospheric conditions, and hence the
water-holding capacity of the atmosphere increases by about 7% for every
1 °C rise in temperature.
[10]
Example
One of
the uses of this equation is to determine if a phase transition will
occur in a given situation. Consider the question of how much pressure
is needed to melt ice at a temperature

below 0 °C. Note that water is unusual in that its change in volume upon melting is negative. We can assume

and substituting in
 = 3.34×105 J/kg (latent heat of fusion for water),
= 3.34×105 J/kg (latent heat of fusion for water), = 273 K (absolute temperature), and
= 273 K (absolute temperature), and = −9.05×10−5 m³/kg (change in specific volume from solid to liquid),
= −9.05×10−5 m³/kg (change in specific volume from solid to liquid),
we obtain
 = −13.5 MPa/K.
= −13.5 MPa/K.
To provide a rough example of how much pressure this is, to melt ice at −7 °C (the temperature many
ice skating rinks are set at) would require balancing a small car (mass = 1000 kg
[11]) on a
thimble (area = 1 cm²).
Second derivative
While
the Clausius–Clapeyron relation gives the slope of the coexistence
curve, it does not provide any information about its curvature or second
derivative. The second derivative of the coexistence curve of phases 1
and 2 is given by
[12]
![{\displaystyle {\begin{aligned}{\frac {\mathrm {d} ^{2}P}{\mathrm {d} T^{2}}}={\frac {1}{v_{2}-v_{1}}}\left[{\frac {c_{p2}-c_{p1}}{T}}-2(v_{2}\alpha _{2}-v_{1}\alpha _{1}){\frac {\mathrm {d} P}{\mathrm {d} T}}\right]+\\{\frac {1}{v_{2}-v_{1}}}\left[(v_{2}\kappa _{T2}-v_{1}\kappa _{T1})\left({\frac {\mathrm {d} P}{\mathrm {d} T}}\right)^{2}\right],\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/79e330791babe59875083305c175a95e06ff78ae)
where subscripts 1 and 2 denote the different phases,

is the specific
heat capacity at constant pressure,

is the
thermal expansion coefficient, and

is the
isothermal compressibility.
 is the slope of the tangent to the coexistence curve at any point,
is the slope of the tangent to the coexistence curve at any point,  is the specific latent heat,
is the specific latent heat,  is the temperature,
is the temperature,  is the specific volume change of the phase transition, and
is the specific volume change of the phase transition, and  is the specific entropy change of the phase transition.
is the specific entropy change of the phase transition. for a homogeneous substance to be a function of specific volume
for a homogeneous substance to be a function of specific volume  and temperature
and temperature  .[3]:508
.[3]:508 is the pressure. Since pressure and temperature are constant, by
definition the derivative of pressure with respect to temperature does
not change.[4][5]:57, 62 & 671 Therefore, the partial derivative of specific entropy may be changed into a total derivative
is the pressure. Since pressure and temperature are constant, by
definition the derivative of pressure with respect to temperature does
not change.[4][5]:57, 62 & 671 Therefore, the partial derivative of specific entropy may be changed into a total derivative to a final phase
to a final phase  ,[3]:508 to obtain
,[3]:508 to obtain and
and  are respectively the change in specific entropy and specific volume. Given that a phase change is an internally reversible process, and that our system is closed, the first law of thermodynamics holds
are respectively the change in specific entropy and specific volume. Given that a phase change is an internally reversible process, and that our system is closed, the first law of thermodynamics holds is the internal energy of the system. Given constant pressure and temperature (during a phase change) and the definition of specific enthalpy
is the internal energy of the system. Given constant pressure and temperature (during a phase change) and the definition of specific enthalpy  , we obtain
, we obtain gives
gives ), we obtain[3]:508[6]
), we obtain[3]:508[6] , at any given point on the curve, to the function
, at any given point on the curve, to the function  of the specific latent heat
of the specific latent heat  , the temperature
, the temperature  , and the change in specific volume
, and the change in specific volume  .
. and
and  , are in contact and at equilibrium with each other. Their chemical potentials are related by
, are in contact and at equilibrium with each other. Their chemical potentials are related by is the specific entropy,
is the specific entropy,  is the specific volume, and
is the specific volume, and  is the molar mass) to obtain
is the molar mass) to obtain greatly exceeds that of the condensed phase
greatly exceeds that of the condensed phase  . Therefore, one may approximate
. Therefore, one may approximate is the pressure,
is the pressure,  is the specific gas constant, and
is the specific gas constant, and  is the temperature. Substituting into the Clapeyron equation
is the temperature. Substituting into the Clapeyron equation is the specific latent heat of the substance.
is the specific latent heat of the substance. and
and  be any two points along the coexistence curve between two phases
be any two points along the coexistence curve between two phases  and
and  . In general,
. In general,  varies between any two such points, as a function of temperature. But if
varies between any two such points, as a function of temperature. But if  is constant,
is constant, is a constant. For a liquid-gas transition,
is a constant. For a liquid-gas transition,  is the specific latent heat (or specific enthalpy) of vaporization; for a solid-gas transition,
is the specific latent heat (or specific enthalpy) of vaporization; for a solid-gas transition,  is the specific latent heat of sublimation. If the latent heat is known, then knowledge of one point on the coexistence curve determines the rest of the curve. Conversely, the relationship between
is the specific latent heat of sublimation. If the latent heat is known, then knowledge of one point on the coexistence curve determines the rest of the curve. Conversely, the relationship between  and
and  is linear, and so linear regression is used to estimate the latent heat.
is linear, and so linear regression is used to estimate the latent heat.is saturation vapor pressure
is temperature
is the specific latent heat of evaporation of water
is the gas constant of water vapor
 , and therefore of the saturation vapor pressure
, and therefore of the saturation vapor pressure  , cannot be neglected in this application. Fortunately, the August-Roche-Magnus formula provides a very good approximation, using pressure in hPa and temperature in Celsius:
, cannot be neglected in this application. Fortunately, the August-Roche-Magnus formula provides a very good approximation, using pressure in hPa and temperature in Celsius: (for which the unit is Celsius). Therefore, the August-Roche-Magnus
equation implies that saturation water vapor pressure changes
approximately exponentially
with temperature under typical atmospheric conditions, and hence the
water-holding capacity of the atmosphere increases by about 7% for every
1 °C rise in temperature.[10]
(for which the unit is Celsius). Therefore, the August-Roche-Magnus
equation implies that saturation water vapor pressure changes
approximately exponentially
with temperature under typical atmospheric conditions, and hence the
water-holding capacity of the atmosphere increases by about 7% for every
1 °C rise in temperature.[10] below 0 °C. Note that water is unusual in that its change in volume upon melting is negative. We can assume
below 0 °C. Note that water is unusual in that its change in volume upon melting is negative. We can assume= 3.34×105 J/kg (latent heat of fusion for water),
= 273 K (absolute temperature), and
= −9.05×10−5 m³/kg (change in specific volume from solid to liquid),
= −13.5 MPa/K.
 is the specific heat capacity at constant pressure,
is the specific heat capacity at constant pressure,  is the thermal expansion coefficient, and
is the thermal expansion coefficient, and  is the isothermal compressibility.
is the isothermal compressibility.




























![{\displaystyle {\begin{aligned}{\frac {\mathrm {d} ^{2}P}{\mathrm {d} T^{2}}}={\frac {1}{v_{2}-v_{1}}}\left[{\frac {c_{p2}-c_{p1}}{T}}-2(v_{2}\alpha _{2}-v_{1}\alpha _{1}){\frac {\mathrm {d} P}{\mathrm {d} T}}\right]+\\{\frac {1}{v_{2}-v_{1}}}\left[(v_{2}\kappa _{T2}-v_{1}\kappa _{T1})\left({\frac {\mathrm {d} P}{\mathrm {d} T}}\right)^{2}\right],\end{aligned}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/79e330791babe59875083305c175a95e06ff78ae)
