From Wikipedia, the free encyclopedia
| Vitamin D | |
|---|---|
| Drug class | |
Cholecalciferol (D3)
|
|
| Use | Rickets, osteoporosis, vitamin D deficiency |
| Biological target | vitamin D receptor |
| ATC code | A11CC |
| External links | |
| MeSH | D014807 |
| AHFS/Drugs.com | MedFacts Natural Products |
Vitamin D refers to a group of fat-soluble secosteroids responsible for enhancing intestinal absorption of calcium, iron, magnesium, phosphate and zinc. In humans, the most important compounds in this group are vitamin D3 (also known as cholecalciferol) and vitamin D2 (ergocalciferol).[1] Cholecalciferol and ergocalciferol can be ingested from the diet and from supplements.[1][2][3] Very few foods contain vitamin D, synthesis of vitamin D (specifically cholecalciferol) in the skin is the major natural sources of the vitamin. Dermal synthesis of vitamin D from cholesterol is dependent on sun exposure (specifically UV-B radiation).
Vitamin D from the diet or dermal synthesis from sunlight is biologically inactive, activation requires enzymatic conversion (hydroxylation) in the liver and kidney. Evidence indicates the synthesis of vitamin D from sun exposure is regulated by a negative feedback loop that prevents toxicity, but because of uncertainty about the cancer risk from sunlight, no recommendations are issued by the Institute of Medicine, USA, for the amount of sun exposure required to meet vitamin D requirements. Accordingly, the Dietary Reference Intake for vitamin D assumes no synthesis occurs and all of a person's vitamin D is from food intake, although that will rarely occur in practice. As vitamin D is synthesized in adequate amounts by most mammals exposed to sunlight, it is not strictly a vitamin, and may be considered a hormone as its synthesis and activity occur in different locations. Vitamin D has a significant role in calcium homeostasis and metabolism. Its discovery was due to effort to find the dietary substance lacking in rickets (the childhood form of osteomalacia).[4]
Beyond its use to prevent osteomalacia or rickets, the evidence for other health effects of vitamin D supplementation in the general population is inconsistent.[5][6] The best evidence of benefit is for bone health.[7] The effect of vitamin D supplementation on mortality is not clear, with one meta-analysis finding a decrease in mortality in elderly people,[8] and another concluding no clear justification exists for recommending vitamin D.[9] Because it found mounting evidence for a benefit to bone health, though it had not found good evidence of other benefits, the Food and Drug Administration of the United States intends to begin requiring manufacturers to declare the amount of Vitamin D on Nutrition Facts labels, as "nutrients of public health significance".[10]
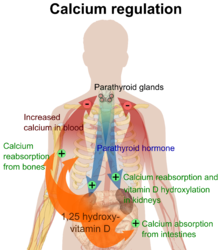
In the liver, cholecalciferol (vitamin D3) is converted to calcidiol, which is also known as calcifediol (INN), 25-hydroxycholecalciferol (aka 25-hydroxyvitamin D3 — abbreviated 25(OH)D3). Ergocalciferol (vitamin D2) is converted in the liver to 25-hydroxyergocalciferol (aka 25-hydroxyvitamin D2 — abbreviated 25(OH)D2). These two specific vitamin D metabolites are measured in serum to determine a person's vitamin D status.[11][12] Part of the calcidiol is converted by the kidneys to calcitriol, the biologically active form of vitamin D.[13] Calcitriol circulates as a hormone in the blood, regulating the concentration of calcium and phosphate in the bloodstream and promoting the healthy growth and remodeling of bone. Calcitriol also affects neuromuscular and immune function.[14]
Types
| Name | Chemical composition | Structure |
|---|---|---|
| Vitamin D1 | molecular compound of ergocalciferol with lumisterol, 1:1 | |
| Vitamin D2 | ergocalciferol (made from ergosterol) |  |
| Vitamin D3 | cholecalciferol (made from 7-dehydrocholesterol in the skin). |  |
| Vitamin D4 | 22-dihydroergocalciferol |  |
| Vitamin D5 | sitocalciferol (made from 7-dehydrositosterol) |  |
Chemically, the various forms of vitamin D are secosteroids, i.e., steroids in which one of the bonds in the steroid rings is broken.[16] The structural difference between vitamin D2 and vitamin D3 is the side chain of D2 contains a double bond between carbons 22 and 23, and a methyl group on carbon 24.
Metabolic activation
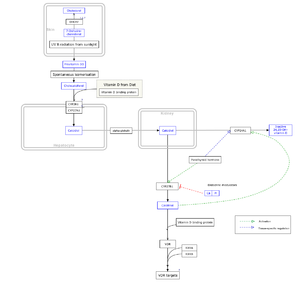
Vitamin D is carried in the bloodstream to the liver, where it is converted into the prohormone calcidiol. Circulating calcidiol may then be converted into calcitriol, the biologically active form of vitamin D, in the kidneys. Following the final converting step in the kidney, calcitriol is released into the circulation. By binding to vitamin D-binding protein, a carrier protein in the plasma, calcitriol is transported to various target organs.[16] In addition to the kidneys, calcitriol is also synthesized by monocyte-macrophages in the immune system. When synthesized by monocyte-macrophages, calcitriol acts locally as a cytokine, defending the body against microbial invaders by stimulating the innate immune system.[18]
Whether it is made in the skin or ingested, cholecalciferol is hydroxylated in the liver at position 25 (upper right of the molecule) to form 25-hydroxycholecalciferol (calcidiol or 25(OH)D). This reaction is catalyzed by the microsomal enzyme vitamin D 25-hydroxylase,[19] which is produced by hepatocytes. Once made, the product is released into the plasma, where it is bound to an α-globulin, vitamin D-binding protein.[20]
Calcidiol is transported to the proximal tubules of the kidneys, where it is hydroxylated at the 1-α position (lower right of the molecule) to form calcitriol (1,25-dihydroxycholecalciferol and abbreviated to 1,25(OH)2D). This product is a potent ligand of the vitamin D receptor, which mediates most of the physiological actions of the vitamin. The conversion of calcidiol to calcitriol is catalyzed by the enzyme 25-hydroxyvitamin D3 1-alpha-hydroxylase, the levels of which are increased by parathyroid hormone (and additionally by low calcium or phosphate).
Deficiency
A diet deficient in vitamin D in conjunction with inadequate sun exposure causes osteomalacia (or rickets when it occurs in children), which is a softening of the bones. In the developed world, this is a rare disease.[21][22] However, vitamin D deficiency has become a worldwide issue in the elderly and remains common in children and adults.[23][24] Low blood calcidiol (25-hydroxy-vitamin D) can result from avoiding the sun.[25] Deficiency results in impaired bone mineralization and bone damage which leads to bone-softening diseases,[26][27] including:Rickets
Rickets, a childhood disease, is characterized by impeded growth and soft, weak, deformed long bones that bend and bow under their weight as children start to walk. This condition is characterized by bow legs,[27] which can be caused by calcium or phosphorus deficiency, as well as a lack of vitamin D; today, it is largely found in low-income countries in Africa, Asia, or the Middle East[28] and in those with genetic disorders such as pseudovitamin D deficiency rickets.[29] Rickets was first described in 1650 by Francis Glisson, who said it had first appeared about 30 years previously in the counties of Dorset and Somerset.[30] In 1857, John Snow suggested rickets, then widespread in Britain, was being caused by the adulteration of bakers' bread with alum.[31] The role of diet in the development of rickets[32][33] was determined by Edward Mellanby between 1918–1920.[34] Nutritional rickets exists in countries with intense year-round sunlight such as Nigeria and can occur without vitamin D deficiency.[35][36] Although rickets and osteomalacia are now rare in Britain, outbreaks have happened in some immigrant communities in which osteomalacia sufferers included women with seemingly adequate daylight outdoor exposure wearing Western clothing.[37] Having darker skin and reduced exposure to sunshine did not produce rickets unless the diet deviated from a Western omnivore pattern characterized by high intakes of meat, fish, and eggs, and low intakes of high-extraction cereals.[38][39][40] The dietary risk factors for rickets include abstaining from animal foods.[37][41] Vitamin D deficiency remains the main cause of rickets among young infants in most countries, because breast milk is low in vitamin D and social customs and climatic conditions can prevent adequate sun exposure. In sunny countries such as Nigeria, South Africa, and Bangladesh, where the disease occurs among older toddlers and children, it has been attributed to low dietary calcium intakes, which are characteristic of cereal-based diets with limited access to dairy products.[40] Rickets was formerly a major public health problem among the US population; in Denver, where ultraviolet rays are about 20% stronger than at sea level on the same latitude,[42] almost two-thirds of 500 children had mild rickets in the late 1920s.[43] An increase in the proportion of animal protein[41][44] in the 20th century American diet coupled with increased consumption of milk[45][46] fortified with relatively small quantities of vitamin D coincided with a dramatic decline in the number of rickets cases.[47] Also, in the United States and Canada, vitamin D-fortified milk, infant vitamin supplements, and vitamin supplements have helped to eradicate the majority of cases of rickets for children with fat malabsorption conditions.[27]Osteomalacia
Osteomalacia is a disease in adults that results from vitamin D deficiency. Characteristics of this disease are softening of the bones, leading to bending of the spine, bowing of the legs, proximal muscle weakness, bone fragility, and increased risk for fractures.[48] Osteomalacia reduces calcium absorption and increases calcium loss from bone, which increases the risk for bone fractures. Osteomalacia is usually present when 25-hydroxyvitamin D levels are less than about 10 ng/mL.[1] The effects of osteomalacia are thought to contribute to chronic musculoskeletal pain,[49][50] There is no persuasive evidence of lower vitamin D levels in chronic pain sufferers.[51]Influence of skin pigmentation
Some research shows dark-skinned people living in temperate climates have lower vitamin D levels.[52][53][53] Dark-skinned people may be less efficient at making vitamin D because melanin in the skin hinders vitamin D synthesis; however, a recent study has found novel evidence that low vitamin D levels among Africans may be due to other reasons.[54] Recent evidence implicates parathyroid hormone in adverse cardiovascular outcomes. Black women have an increase in serum parathyroid hormone at a lower 25(OH)D level than white women.[55] A large-scale association study of the genetic determinants of vitamin D insufficiency in Caucasians found no links to pigmentation.[56][57]However, the uniform occurrence of low serum 25(OH)D in Indians living in India[58] and Chinese in China,[59] does not support the hypothesis that the low levels seen in the more pigmented are due to lack of synthesis from the sun at higher latitudes.
Excess
Vitamin D toxicity is rare.[24] It is caused by supplementing with high doses of vitamin D rather than sunlight. The threshold for vitamin D toxicity has not been established; however, the tolerable upper intake level (UL), according to some research, is 4,000 IU/day for ages 9–71.[7] Whereas another research concludes that in healthy adults, sustained intake of more than 1250 μg/day (50,000 IU) can produce overt toxicity after several months and can increase serum 25-hydroxyvitamin D levels to 150 ng/ml and greater;[24][60] those with certain medical conditions, such as primary hyperparathyroidism,[61] are far more sensitive to vitamin D and develop hypercalcemia in response to any increase in vitamin D nutrition, while maternal hypercalcemia during pregnancy may increase fetal sensitivity to effects of vitamin D and lead to a syndrome of mental retardation and facial deformities.[61][62]Hypercalcemia is a strong indication of vitamin D toxicity, noted with an increase in urination and thirst. If hypercalcemia is not treated, it results in excess deposits of calcium in soft tissues and organs such as the kidneys, liver, and heart, resulting in pain and organ damage.[24][27][48] Pregnant or breastfeeding women should consult a doctor before taking a vitamin D supplement. The FDA advised manufacturers of liquid vitamin D supplements that droppers accompanying these products should be clearly and accurately marked for 400 international units (IU). In addition, for products intended for infants, the FDA recommends the dropper hold no more than 400 IU.[63] For infants (birth to 12 months), the tolerable upper limit (maximum amount that can be tolerated without harm) is set at 25 μg/day (1,000 IU). One thousand micrograms per day in infants has produced toxicity within one month.[60] After being commissioned by the Canadian and American governments, the Institute of Medicine (IOM) as of 30 November 2010[update], has increased the tolerable upper limit (UL) to 2,500 IU per day for ages 1–3 years, 3,000 IU per day for ages 4–8 years and 4,000 IU per day for ages 9–71+ years (including pregnant or lactating women).[7]
Vitamin D overdose causes hypercalcemia, and the main symptoms of vitamin D overdose are those of hypercalcemia: anorexia, nausea, and vomiting can occur, frequently followed by polyuria, polydipsia, weakness, insomnia, nervousness, pruritus, and, ultimately, renal failure. Proteinuria, urinary casts, azotemia, and metastatic calcification (especially in the kidneys) may develop.[60] Other symptoms of vitamin D toxicity include mental retardation in young children, abnormal bone growth and formation, diarrhea, irritability, weight loss, and severe depression.[24][48] Vitamin D toxicity is treated by discontinuing vitamin D supplementation and restricting calcium intake. Kidney damage may be irreversible. Exposure to sunlight for extended periods of time does not normally cause vitamin D toxicity.[61] Within about 20 minutes of ultraviolet exposure in light-skinned individuals (three to six times longer for pigmented skin), the concentrations of vitamin D precursors produced in the skin reach an equilibrium, and any further vitamin D produced is degraded.[64]
Published cases of toxicity involving hypercalcemia in which the vitamin D dose and the 25-hydroxy-vitamin D levels are known all involve an intake of ≥40,000 IU (1,000 μg) per day.[61] Recommending supplementation, when those supposedly in need of it are labeled healthy, has proved contentious, and doubt exists concerning long-term effects of attaining and maintaining high serum 25(OH)D by supplementation.[65]
Health effects of supplementation
The effects of vitamin D supplementation on health are uncertain.[6][66] A 2013 review did not find any effect from supplementation on the rates of disease, other than a tentative decrease in mortality in the elderly.[67] Low vitamin D levels may result from disease rather than cause disease.[67]A United States Institute of Medicine (IOM) report states: "Outcomes related to cancer, cardiovascular disease and hypertension, and diabetes and metabolic syndrome, falls and physical performance, immune functioning and autoimmune disorders, infections, neuropsychological functioning, and preeclampsia could not be linked reliably with calcium or vitamin D intake and were often conflicting."[7]:5 Some researchers claim the IOM was too definitive in its recommendations and made a mathematical mistake when calculating the blood level of vitamin D associated with bone health.[68] Members of the IOM panel maintain that they used a "standard procedure for dietary recommendations" and that the report is solidly based on the data. Research on vitamin D supplements, including large-scale clinical trials, is continuing.[68]
Mortality
Vitamin D3 supplementation has been tentatively found to lead to a reduced risk of death in the elderly,[8][67] but the effect has not been deemed pronounced or certain enough to make taking supplements recommendable.[9]Other forms (Vitamin D2, alfacalcidol, and calcitriol) do not appear to have any beneficial effects with regards to the risk of death.[8] High blood levels appear to be associated with a lower risk of death, but it is unclear if supplementation can result in this benefit.[69] Both an excess and a deficiency in vitamin D appear to cause abnormal functioning and premature aging.[70][71][72] The relationship between serum calcidiol level and all-cause mortality is parabolic.[7] Harm from vitamin D appears to occur at a lower vitamin D level in the black population than in the white population.[7]:435
Bone health
In general, no good evidence supports the commonly held belief that vitamin D supplements can help prevent osteoporosis.[9] Its general use for prevention of this disease in those without vitamin D deficiency is thus likely not needed.[73]For older people with osteoporosis, taking vitamin D with calcium may help prevent hip fractures, but it also slightly increases the risk of stomach and kidney problems.[74] Supplementation with higher doses of vitamin D, in those older than 65 years, may decrease fracture risk.[75] This appears to apply more to people in institutions than those living independently.[76]
Vitamin D deficiency causes osteomalacia (called rickets when it occurs in children). Use of vitamin D in children with normal vitamin D levels does not appear to improve bone density.[77] Beyond that, low serum vitamin D levels have been associated with falls, and low bone mineral density.[78] Taking extra vitamin D; however, does not appear to change the risk.[79]
Athletes who are vitamin D deficient are at an increased risk of stress fractures and/or major breaks, particularly those engaging in contact sports. The greatest benefit with supplementation is seen in athletes who are deficient (25(OH)D serum levels <30 ng/ml), or severely deficient (25(OH)D serum levels <25 ng/ml). Incremental decreases in risks are observed with rising serum 25(OH)D concentrations plateauing at 50 ng/ml with no additional benefits seen in levels beyond this point.[80]
Cancer
Vitamin D supplements have been widely marketed on the internet and elsewhere for their claimed anticancer properties,[81] but taking vitamin D supplements has been found to have no significant effect on cancer risk.[9] Some research has suggested that vitamin D3 decreases the risk of death from cancer, but concerns with the quality of the data were noted.[82]Insufficient evidence exists to recommend vitamin D to be prescribed for people with cancer, although some evidence suggests hypovitaminosis D may be associated with a worse outcome for some cancers,[83] and that higher 25-hydroxy vitamin D levels at the time of diagnosis are associated with better outcomes.[84]
Cardiovascular disease
Taking vitamin D supplements does not meaningfully reduce the risk of stroke, cerebrovascular disease, cardial infarction, or ischaemic heart disease.[9]Depression
Clinical trials of vitamin D supplementation for depressive symptoms have generally been of low quality and show no overall effect, although subgroup analysis showed supplementation for participants with clinically significant depressive symptoms or depressive disorder had a moderate effect.[85]Cognition and dementia
A systematic review of clinical studies shows an association between low vitamin D levels, cognitive impairment, and a higher risk of developing Alzheimer's disease. However, lower vitamin D concentrations is also associated with poor nutrition and spending less time outdoors. Therefore alternative explanations for the increase in cognitive impairment exist and hence a direct causal relationship between vitamin D levels and cognition could not be established.[86]Immune system
Infectious disease
In general, vitamin D functions to activate the innate and dampen the adaptive immune systems.[87] Deficiency has been linked to increased risk of viral infections, including HIV and influenza.[88][89][90] Low levels of vitamin D appear to be a risk factor for tuberculosis,[91] and historically it was used as a treatment.[92]Autoimmune disease
Although tentative data link low levels of vitamin D to asthma, evidence to support a beneficial effect from supplementation is inconclusive.[93] Accordingly, supplementation is not currently recommended for treatment or prevention of asthma.[94]Vitamin D hypovitaminosis may be a risk factor for multiple sclerosis,[95] but no evidence indicates vitamin D has any clinically significant benefit as a treatment.[96] Further research is needed to determine if the association represents a cause and effect relationship.[97]
Pregnancy
Low levels of vitamin D in pregnancy are associated with gestational diabetes, pre-eclampsia, and small infants.[98] The benefit of supplements, however, is unclear.[98] Pregnant women who take an adequate amount of vitamin D during gestation may experience positive immune effects.[99] Pregnant women often do not take the recommended amount of vitamin D.[99]Mechanism of action
Biosynthesis
In the presence of UV radiation, many animals synthesize vitamin D3 from 7-dehydrocholesterol, and many fungi synthesize vitamin D2 from ergosterol.Photochemistry
The transformation that converts 7-dehydrocholesterol to vitamin D3 occurs in two steps.[100][101] First, 7-dehydrocholesterol is photolyzed by ultraviolet light in a 6-electron conrotatory ring-opening electrocyclic reaction; the product is previtamin D3. Second, previtamin D3 spontaneously isomerizes to vitamin D3 (cholecalciferol) in an antarafacial sigmatropic [1,7] hydride shift. At room temperature, the transformation of previtamin D3 to vitamin D3 in an organic solvent takes about 12 days to complete. The conversion of previtamin D3 to vitamin D3 in the skin is about 10 times faster than in an organic solvent [102]
Evolution
Photosynthesis of vitamin D in the ocean by phytoplankton (such as coccolithophore and Emiliania huxleyi) has existed for more than 500 million years and continues to the present. Although primitive vertebrates in the ocean could absorb calcium from the ocean into their skeletons and eat plankton rich in vitamin D, land animals required another way to satisfy their vitamin D requirement for a calcified skeleton without relying on plants. Land vertebrates have been making their own vitamin D for more than 350 million years.[103]Vitamin D can be synthesized only by a photochemical process, so land vertebrates had to ingest foods that contained vitamin D or had to be exposed to sunlight to photosynthesize vitamin D in their skin to satisfy their vitamin D requirements.[102]
Synthesis in the skin
Vitamin D3 is produced photochemically in the skin from 7-dehydrocholesterol. The precursor of vitamin D3, 7-dehydrocholesterol is produced in relatively large quantities. 10,000 to 20,000 IU of vitamin D are produced in 30 minutes of whole-body exposure, in the skin of most vertebrate animals, including humans.[104] 7-Dehydrocholesterol reacts with UVB light at wavelengths between 270 and 300 nm, with peak synthesis occurring between 295 and 297 nm.[105] These wavelengths are present in sunlight, as well as in the light emitted by the UV lamps in tanning beds (which produce ultraviolet primarily in the UVA spectrum, but typically produce 4% to 10% of the total UV emissions as UVB). Vitamin D3 can be made in the skin. Exposure to light through windows is insufficient because glass almost completely blocks UVB light.[106][107]
Depending on the intensity of UVB rays and the minutes of exposure, an equilibrium can develop in the skin, and vitamin D degrades as fast as it is generated.[64]
The skin consists of two primary layers: the inner layer called the dermis, composed largely of connective tissue, and the outer, thinner epidermis. Thick epidermis in the soles and palms consists of five strata; from outer to inner, they are: the stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and stratum basale.
Vitamin D is produced in the two innermost strata, the stratum basale and stratum spinosum.
The naked mole-rat appears to be naturally cholecalciferol-deficient, as serum 25-OH vitamin D levels are undetectable.[108] In some animals, the presence of fur or feathers blocks the UV rays from reaching the skin. In birds and fur-bearing mammals, vitamin D is generated from the oily secretions of the skin deposited onto the feathers or fur and is obtained orally during grooming.[109]
Sunscreen
Sunscreen absorbs or reflects ultraviolet light and prevents much of it from reaching the skin. Sunscreen with a sun protection factor (SPF) of 8 based on the UVB spectrum has been reported to decrease vitamin D synthetic capacity by 95%, whereas sunscreen with an SPF of 15 can reduce synthetic capacity by 98%.[110]Biological activity
The active vitamin D metabolite calcitriol mediates its biological effects by binding to the vitamin D receptor (VDR), which is principally located in the nuclei of target cells.[16] The binding of calcitriol to the VDR allows the VDR to act as a transcription factor that modulates the gene expression of transport proteins (such as TRPV6 and calbindin), which are involved in calcium absorption in the intestine.[111] The vitamin D receptor belongs to the nuclear receptor superfamily of steroid/thyroid hormone receptors, and VDRs are expressed by cells in most organs, including the brain, heart, skin, gonads, prostate, and breast. VDR activation in the intestine, bone, kidney, and parathyroid gland cells leads to the maintenance of calcium and phosphorus levels in the blood (with the assistance of parathyroid hormone and calcitonin) and to the maintenance of bone content.[47]
One of the most important roles of vitamin D is to maintain skeletal calcium balance by promoting calcium absorption in the intestines, promoting bone resorption by increasing osteoclast number, maintaining calcium and phosphate levels for bone formation, and allowing proper functioning of parathyroid hormone to maintain serum calcium levels. Vitamin D deficiency can result in lower bone mineral density and an increased risk of reduced bone density (osteoporosis) or bone fracture because a lack of vitamin D alters mineral metabolism in the body.[112] Thus, although it may initially appear paradoxical, vitamin D is also critical for bone remodeling through its role as a potent stimulator of bone resorption.[113]
The VDR is known to be involved in cell proliferation and differentiation. Vitamin D also affects the immune system, and VDRs are expressed in several white blood cells, including monocytes and activated T and B cells.[114] Vitamin D increases expression of the tyrosine hydroxylase gene in adrenal medullary cells. It also is involved in the biosynthesis of neurotrophic factors, synthesis of nitric oxide synthase, and increased glutathione levels.[115]
Apart from VDR activation, various alternative mechanisms of action are known. An important one of these is its role as a natural inhibitor of signal transduction by hedgehog (a hormone involved in morphogenesis).[116][117]
History
American researchers Elmer McCollum and Marguerite Davis in 1914[4] discovered a substance in cod liver oil which later was called "vitamin A". British doctor Edward Mellanby noticed dogs that were fed cod liver oil did not develop rickets and concluded vitamin A, or a closely associated factor, could prevent the disease. In 1922, Elmer McCollum tested modified cod liver oil in which the vitamin A had been destroyed.[4] The modified oil cured the sick dogs, so McCollum concluded the factor in cod liver oil which cured rickets was distinct from vitamin A. He called it vitamin D because it was the fourth vitamin to be named.[118][119][120] It was not initially realized that, unlike other vitamins, vitamin D can be synthesised by humans through exposure to UV light.In 1925,[4] it was established that when 7-dehydrocholesterol is irradiated with light, a form of a fat-soluble vitamin is produced (now known as D3). Alfred Fabian Hess stated, "light equals vitamin D."[121] Adolf Windaus, at the University of Göttingen in Germany, received the Nobel Prize in Chemistry in 1928 for his work on the constitution of sterols and their connection with vitamins.[122] In 1929, a group at NIMR in Hampstead, London, were working on the structure of vitamin D, which was still unknown, as well as the structure of steroids. A meeting took place with J.B.S. Haldane, J.D. Bernal and Dorothy Crowfoot to discuss possible structures, which contributed to bringing a team together. X-ray crystallography demonstrated the sterol molecules were flat, not as proposed by the German team led by Windaus. In 1932, Otto Rosenheim and Harold King published a paper putting forward structures for sterols and bile acids which found immediate acceptance.[123] The informal academic collaboration between the team members Robert Benedict Bourdillon, Otto Rosenheim, Harold King, and Kenneth Callow was very productive and led to the isolation and characterization of vitamin D.[124] At this time, the policy of the Medical Research Council was not to patent discoveries, believing the results of medical research should be open to everybody. In the 1930s, Windaus clarified further the chemical structure of vitamin D.[125]
In 1923, American biochemist Harry Steenbock at the University of Wisconsin demonstrated that irradiation by ultraviolet light increased the vitamin D content of foods and other organic materials.[126] After irradiating rodent food, Steenbock discovered the rodents were cured of rickets. A vitamin D deficiency is a known cause of rickets. Using $300 of his own money, Steenbock patented his invention. His irradiation technique was used for foodstuffs, most memorably for milk. By the expiration of his patent in 1945, rickets had been all but eliminated in the US.[127]
In 1971–72, the further metabolism of vitamin D to active forms was discovered. In the liver, vitamin D was found to be converted to calcidiol.[12][128] Part of the calcidiol is then converted by the kidneys to calcitriol, the biologically active form of vitamin D.[13] Calcitriol circulates as a hormone in the blood, regulating the concentration of calcium and phosphate in the bloodstream and promoting the healthy growth and remodeling of bone. Both calcidiol and calcitriol were identified by a team led by Michael F. Holick in the laboratory of Hector DeLuca.[13][129]
Society and culture
Recommendations
Dietary reference intakes
Different institutions propose different recommendations concerning daily amounts of the vitamin. Commonly recommended daily intake of vitamin D is not sufficient if sunlight exposure is limited.[130](Conversion : 1 µg = 40 IU and 0.025 µg = 1 IU)[131]
Australia and New Zealand
About a third of Australians have vitamin D deficiency.[132] Australia and New Zealand have established guidelines for dietary vitamin D intake as follows:[133]| Age group | Adequate Intake (μg) | Upper Level of Intake (μg) |
|---|---|---|
| Infants 0–12 months | 5.0 | 25.0 |
| Children 1–18 years | 5.0 | 80.0 |
| Adults 19–50 years | 5.0 | 80.0 |
| Adults 51–70 years | 10.0 | 80.0 |
| Adults > 70 years | 15.0 | 80.0 |
Canada
According to Health Canada[134] the recommended dietary allowances (RDA) for vitamin D are:| Age group | RDA (IU) | Tolerable upper intake (IU) |
|---|---|---|
| Infants 0–6 months | 400* | 1,000 |
| Infants 7–12 months | 400* | 1,500 |
| Children 1–3 years | 600 | 2,500 |
| Children 4–8 years | 600 | 3,000 |
| Children and Adults 9–70 years | 600 | 4,000 |
| Adults > 70 years | 800 | 4,000 |
| Pregnancy & Lactation | 600 | 4,000 |
European Union
The recommended daily amount for vitamin D in the European Union is 5 µg.[135] In 2012, the German Society for Nutrition, a private organisation, increased the recommended daily amount to 20 µg.[136]The European Menopause and Andropause Society recommended 15 µg (600 IU) until age 70, and 20 µg (800 IU) in older than 71 years, in postmenopausal women. This dose should be increased to 4,000 IU/day in some patients with very low vitamin D status or in case of comorbid conditions.[137]
The UK National Health Service recommends babies and young children aged six months to five years, pregnant or breastfeeding women, and sun-deprived elderly people should take daily vitamin supplements to ensure sufficient vitamin D intake; the general population gets enough vitamin D from good diets and from sunlight.[138]
United States
According to the United States Institute of Medicine,[7] the recommended dietary allowances (RDA) of vitamin D are:| Age group | RDA (IU/day) |
|---|---|
| Infants 0–6 months | 400* |
| Infants 6–12 months | 400* |
| 1–70 years | 600 (15 μg/day) |
| 71+ years | 800 (20 μg/day) |
| Pregnant/Lactating | 600 (15 μg/day) |
- Asterisk for infants indicates adequate intake (AI) for infants, as an RDA has yet to be established for infants.[7]
Upper intake levels
The tolerable upper intake level is defined as "the highest average daily intake of a nutrient that is likely to pose no risk of adverse health effects for nearly all persons in the general population.[7]:403 " Although tolerable upper intake levels are believed to be safe, information on the long-term effects is incomplete and these levels of intake are not recommended:[7]:403:433| Age group | Tolerable upper intake level |
|---|---|
| Infants 0–6 months | 1,000 IU/day (25 µg/day) |
| Infants 6–12 months | 1,500 IU/day (37.5 µg/day) |
| 1–3 years | 2,500 IU/day (62.5 µg/day) |
| 4–8 years | 3,000 IU/day (75 µg/day) |
| 9+ years | 4,000 IU/day (100 µg/day) |
| Pregnant/lactating | 4,000 IU/day[7]:5(100 µg/day) |
The dietary reference intake for vitamin D issued by the Institute of Medicine (IOM) in 2010 superseded a previous recommendation which had adequate intake status. The recommendations were formed assuming the individual has no skin synthesis of vitamin D because of inadequate sun exposure. The reference intake for vitamin D refers to total intake from food, beverages and supplements, is intended for the North American population, and assumes that calcium requirements are being met.[7]:5
One school of thought contends the human physiology is fine-tuned to an intake of 4,000–12,000 IU/day from sun exposure with concomitant serum 25-hydroxyvitamin D levels of 40 to 80 ng/ml[139] and this is required for optimal health. Proponents of this view, who include some members of the panel that drafted a now-superseded 1997 report on vitamin D from the IOM, contend the IOM's warning about serum concentrations above 50 ng/ml lacks biological plausibility. They suggest, for some people, reducing the risk of preventable disease requires a higher level of vitamin D than that recommended by the IOM.[139][140]
According to the European Food Safety Authority, the tolerable upper intake levels[141] are:
- 0–12 months: 25 µg/day (1,000 IU)
- 1–10 years: 50 µg/day (2,000 IU)
- 11–17 years: 100 µg/day (4,000 IU)
- 17+: 100 µg/day (4,000 IU)
- Pregnant/lactating women: 100 µg/day (4,000 IU)
Serum 25-hydroxyvitamin D
US labs generally report 25(OH)D levels as ng/ml. Other countries often use nmol/l.An IOM committee concluded a serum 25-hydroxyvitamin D level of 20 ng/ml (50 nmol/l) is desirable for bone and overall health. The dietary reference intakes for vitamin D are chosen with a margin of safety and 'overshoot' the targeted serum value to ensure the specified levels of intake achieve the desired serum 25-hydroxyvitamin D levels in almost all persons. No contributions to serum 25-hydroxyvitamin D level are assumed from sun exposure and the recommendations are fully applicable to people with dark skin or negligible exposure to sunlight.
The Institute found serum 25-hydroxyvitamin D concentrations above 30 ng/ml (75 nmol/l) are "not consistently associated with increased benefit". Serum 25-hydroxyvitamin D levels above 50 ng/ml (125 nmol/l) may be cause for concern.[7] However, the desired range of serum 25-hydroxyvitamin D is between 20 and 50 ng/ml.[7]
The risk of cardiovascular disease is lower when vitamin D ranged from 20 to 60 nmol/l (8 to 24 ng/ml). A "threshold effect" appears to occur once a level of 60 nmol/l (24 ng/ml) has been reached i.e., levels of vitamin D over 60 nmol/l did not show added benefit.[142]
Allowable health claims
Apart from the above discussion on health effects or scientific evidence for lowering disease risk, governmental regulatory agencies stipulate for the food industry health claims allowable as statements on packaging.European Food Safety Authority (EFSA)[143]
- normal function of the immune system
- normal inflammatory response
- normal muscle function
- reduced risk of falling in people over age 60[144]
- may reduce the risk of osteoporosis[145]
- adequate calcium and regular exercise may help to achieve strong bones in children and adolescents and may reduce the risk of osteoporosis in older adults. An adequate intake of vitamin D is also necessary[146]
Dietary sources
Vitamin D is found in few dietary sources.[1][3][24][27] Sunlight exposure is the primary source of vitamin D for majority of people, other than supplements.[2]Vitamin D2
Fungus, from USDA nutrient database (per 100 g):[149] Low values in mushrooms for vitamin D below indicate incidental exposure to sunlight which activates synthesis of vitamin D2. When fresh mushrooms or dried powders are purposely exposed to artificial sunlight by use of an industrial ultraviolet lamp, vitamin D levels can be controlled at much higher levels.[150][151]- Mushrooms, portabella, exposed to ultraviolet light, raw: Vitamin D2: 11.2 μg (446 IU)
- Mushrooms, portabella, exposed to ultraviolet light, grilled: Vitamin D2: 13.1 μg (524 IU)
- Mushrooms, shiitake, dried: Vitamin D2: 3.9 μg (154 IU)
- Mushrooms, shiitake, raw: Vitamin D2: 0.4 μg (18 IU)
- Mushrooms, portabella, raw: Vitamin D2: 0.3 μg (10 IU)
- Mushroom powder, any species, illuminated with sunlight or artificial ultraviolet light sources
Human bioavailability of vitamin D2 from vitamin D2-enhanced button mushrooms via UV-B irradiation is effective in improving vitamin D status and not different from a vitamin D2 supplement.[153] Vitamin D2 from UV-irradiated yeast baked into bread or mushrooms is bioavailable and increases blood levels of 25(OH)D.[151][154]
By visual assessment or using a chromometer, no significant discoloration of irradiated mushrooms, as measured by the degree of "whiteness", was observed.[155] Claims have been made that a normal serving (approx. 3 oz or 1/2 cup, or 60 grams) of fresh mushrooms treated with ultraviolet light have increased vitamin D content to levels up to 80 micrograms or 2700 IU if exposed to just 5 minutes of UV light after being harvested.[150]
Plants
- Alfalfa (Medicago sativa subsp. sativa), shoot: 4.8 μg (192 IU) vitamin D2, 0.1 μg (4 IU) vitamin D3 (per 100 g).[156]
Vitamin D3
In some countries, staple foods are artificially fortified with vitamin D.[157]- Vegan sources
- Lichen
- Cladina arbuscula specimens grown under different natural conditions: The contents of vitamin D3 range from 0.67 to 2.04 μg g⁻¹ dry matter in the thalli of C. arbuscula specimens grown under different natural conditions.[158]
- Lichen
- Animal sources[149]
- Fish liver oils, such as cod liver oil, 4.5 g (1 teaspoon) provides 450 IU (100 IU/g)
- Fatty fish species, such as:
- Salmon, cooked, 85 g (3 oz) provides 444 IU (5.2 IU/g)
- Mackerel, cooked, 85 g, 390 IU (4.6 IU/g)
- Tuna, canned in oil, 100 g, 269 IU (2.7 IU/g)
- Sardines, canned in oil, drained, 100 g (3.5 oz), 193 IU (1.9 IU/g)
- Cooked egg yolk provides 44 IU if egg weighs 61 g (0.7 IU/g)
- Beef liver, cooked, 85 g, provides 42 IU (0.5 IU/g)