Antibiotic resistance tests: Bacteria are streaked on dishes with white
disks, each impregnated with a different antibiotic. Clear rings, such
as those on the left, show that bacteria have not grown—indicating that
these bacteria are not resistant. The bacteria on the right are fully
susceptible to only three of the seven antibiotics tested.[1]
Antimicrobial resistance (AMR or AR) is the ability of a microbe to resist the effects of medication previously used to treat them.[2][3][4] The term includes the more specific antibiotic resistance (AR or ABR), which applies only to bacteria becoming resistant to antibiotics.[3] Resistant microbes are more difficult to treat, requiring alternative medications or higher doses, both of which may be more expensive or more toxic. Microbes resistant to multiple antimicrobials are called multidrug resistant (MDR); those extensively drug resistant (XDR) or totally drug resistant (TDR) are sometimes called "superbugs".[5]
Resistance arises through one of three mechanisms: natural resistance in certain types of bacteria, genetic mutation, or by one species acquiring resistance from another.[6] All classes of microbes can develop resistance: fungi develop antifungal resistance, viruses develop antiviral resistance, protozoa develop antiprotozoal resistance, and bacteria develop antibiotic resistance. Resistance can appear spontaneously because of random mutations.
Preventive measures include only using antibiotics when needed, thereby stopping misuse of antibiotics or antimicrobials.[7][8] Narrow-spectrum antibiotics are preferred over broad-spectrum antibiotics when possible, as effectively and accurately targeting specific organisms is less likely to cause resistance.[9] For people who take these medications at home, education about proper use is essential. Health care providers can minimize spread of resistant infections by use of proper sanitation and hygiene, including handwashing and disinfecting between patients, and should encourage the same of the patient, visitors, and family members.[10]
Rising drug resistance is caused mainly by use of antimicrobials in humans and other animals, and spread of resistant strains between the two.[7] Growing resistance has also been linked to dumping of inadequately treated effluents from the pharmaceutical industry, especially in countries where bulk drugs are manufactured.[11] Antibiotics increase selective pressure in bacterial populations, causing vulnerable bacteria to die; this increases the percentage of resistant bacteria which continue growing. With resistance to antibiotics becoming more common there is greater need for alternative treatments. Calls for new antibiotic therapies have been issued, but new drug development is becoming rarer.[12]
Antimicrobial resistance is on the rise globally, predominantly due to greater access to antibiotic drugs in developing countries.[13] Estimates are that 700,000 to several million deaths result per year.[14][15] Each year in the United States, at least 2 million people become infected with bacteria that are resistant to antibiotics and at least 23,000 people die as a result.[16] There are public calls for global collective action to address the threat include proposals for international treaties on antimicrobial resistance.[17] Worldwide antibiotic resistance is not fully mapped, but poorer countries with weak healthcare systems are more affected.[8]
Definition
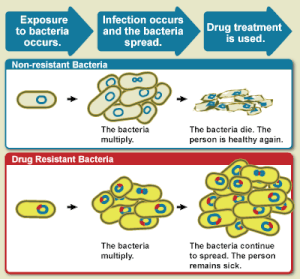
Diagram showing the difference between non-resistant bacteria and drug
resistant bacteria. Non-resistant bacteria multiply, and upon drug
treatment, the bacteria die. Drug resistant bacteria multiply as well,
but upon drug treatment, the bacteria continue to spread.[18]
The WHO defines antimicrobial resistance as a microorganism's resistance to an antimicrobial drug that was once able to treat an infection by that microorganism.[3] A person cannot become resistant to antibiotics. Resistance is a property of the microbe, not a person or other organism infected by a microbe.[19]
Overview
A World Health Organization (WHO) report released April 2014 stated, "this serious threat is no longer a prediction for the future, it is happening right now in every region of the world and has the potential to affect anyone, of any age, in any country. Antibiotic resistance—when bacteria change so antibiotics no longer work in people who need them to treat infections—is now a major threat to public health."[20]Causes
How antibiotic resistance evolves and spreads
Bacteria with resistance to antibiotics predate medical use of antibiotics by humans.[21][22] However, widespread antibiotic use has made more bacteria resistant through the process of evolutionary pressure.[23][24]
Reasons for the widespread use of antibiotics in human medicine include:
- increasing global availability over time since the 1950s
- uncontrolled sale in many low or middle income countries, where they can be obtained over the counter without a prescription, potentially resulting in antibiotics being used when not indicated.[25]:1060 This may result in emergence of resistance in any remaining bacteria.
- Antibiotic use in livestock feed at low doses for growth promotion is an accepted practice in many industrialized countries and is known to lead to increased levels of resistance.[26][27]
- Releasing large quantities of antibiotics into the environment during pharmaceutical manufacturing through inadequate wastewater treatment increases the risk that antibiotic-resistant strains will develop and spread.[28][29]
- It is uncertain whether antibacterials in soaps and other products contribute to antibiotic resistance, but antibacterial soaps are discouraged for other reasons.[30][31]
Human medicine
Deaths attributable to antimicrobial resistance every year compared to other major causes of death.[15]
Increasing bacterial resistance is linked with the volume of antibiotic prescribed, as well as missing doses when taking antibiotics.[32] Inappropriate prescribing of antibiotics has been attributed to a number of causes, such as: patients insisting on antibiotics and physicians prescribing them as they do not have time to explain why they are not necessary. Another cause can be physicians not knowing when to prescribe antibiotics or being overly cautious for medical or legal reasons.[33] For example, 70 to 80 percent of diarrhea is caused by viral pathogens, for which antibiotics are not effective. But nevertheless, around 40 percent of these cases are attempted to be treated with antibiotics.[34] In some areas even over 80 percent of such cases are attempted to be treated with antibiotics.[34]
Lower antibiotic concentration contributes to the increase of AMR by introducing more mutations that support bacterial growth in higher antibiotic concentration. For example, sub-inhibitory concentration have induced genetic mutation in bacteria such as Pseudomonas aeruginosa and Bacteroides fragilis.[35]
Up to half of antibiotics used in humans are unnecessary and inappropriate.[7] For example, a third of people believe that antibiotics are effective for the common cold,[36] and the common cold is the most common reason antibiotics are prescribed even though antibiotics are useless against viruses.[37] A single regimen of antibiotics even in compliant individuals leads to a greater risk of resistant organisms to that antibiotic in the person for a month to possibly a year.[38][39]
Antibiotic resistance increases with duration of treatment. Therefore, as long as an effective minimum is kept, shorter courses of antibiotics are likely to decrease rates of resistance, reduce cost, and have better outcomes with fewer complications.[9] Short course regimens exist for community-acquired pneumonia[40] spontaneous bacterial peritonitis,[41] suspected lung infections in intense care wards,[42] so-called acute abdomen,[43] middle ear infections, sinusitis and throat infections,[44] and penetrating gut injuries.[45][46] In some situations a short course may not cure the infection as well as a long course.[47] A BMJ editorial recommended that antibiotics can often be safely stopped 72 hours after symptoms resolve.[48]
Because individuals may feel better before the infection is eradicated, doctors must provide instructions to them so they know when it is safe to stop taking a prescription. Some researchers advocate doctors' using a very short course of antibiotics, reevaluating the patient after a few days, and stopping treatment if there are no clinical signs of infection.[49]
Certain antibiotic classes result in resistance more than others. Increased rates of MRSA infections are seen when using glycopeptides, cephalosporins, and quinolone antibiotics.[50][51] Cephalosporins, and particularly quinolones and clindamycin, are more likely to produce colonisation with Clostridium difficile.[52][53]
Factors within the intensive care unit setting such as mechanical ventilation and multiple underlying diseases also appear to contribute to bacterial resistance.[54] Poor hand hygiene by hospital staff has been associated with the spread of resistant organisms.[55]
Veterinary medicine

All animals carry bacteria in their intestines. Antibiotics are given to
animals. Antibiotics kill most bacteria. But resistant bacteria survive
and multiply.
The World Health Organization concluded that inappropriate use of antibiotics in animal husbandry is an underlying contributor to the emergence and spread of antibiotic-resistant germs, and that the use of antibiotics as growth promoters in animal feeds should be restricted.[56] The World Organisation for Animal Health has added to the Terrestrial Animal Health Code a series of guidelines with recommendations to its members for the creation and harmonization of national antimicrobial resistance surveillance and monitoring programs,[57] monitoring of the quantities of antibiotics used in animal husbandry,[58] and recommendations to ensure the proper and prudent use of antibiotic substances. Another guideline is to implement methodologies that help to establish associated risk factors and assess the risk of antibiotic resistance.[59]
Natural occurrence
Naturally occurring antibiotic resistance is common.[60] Genes for resistance to antibiotics, like antibiotics themselves, are ancient.[61][62] The genes that confer resistance are known as the environmental resistome.[60] These genes may be transferred from non-disease-causing bacteria to those that do cause disease, leading to clinically significant antibiotic resistance.[60]In 1952 it was shown that penicillin-resistant bacteria existed before penicillin treatment;[63] and also preexistent bacterial resistance to streptomycin.[64] In 1962, the presence of penicillinase was detected in dormant endospores of Bacillus licheniformis, revived from dried soil on the roots of plants, preserved since 1689 in the British Museum.[65][66][67] Six strains of Clostridium, found in the bowels of William Braine and John Hartnell (members of the Franklin Expedition) showed resistance to cefoxitin and clindamycin.[68]
Penicillinase may have emerged as a defense mechanism for bacteria in their habitats, such as the case of penicillinase-rich Staphylococcus aureus, living with penicillin-producing Trichophyton; however, this may be circumstantial.[67] Search for a penicillinase ancestor has focused on the class of proteins that must be a priori capable of specific combination with penicillin.[69] The resistance to cefoxitin and clindamycin in turn was attributed to Braine's and Hartnell's contact with microorganisms that naturally produce them or random mutation in the chromosomes of Clostridium strains.[68]
There is evidence that heavy metals and other pollutants may select for antibiotic-resistant bacteria, generating a constant source of them in small numbers.[70]
Water pollution
Antibiotic resistance is a growing problem among humans and wildlife in terrestrial or aquatic environments. In this respect, the spread and contamination of the environment, especially through water pollution "hot spots" such as hospital wastewater and untreated urban wastewater, is a growing and serious public health problem.[71][72] Antibiotics have been polluting the environment since their introduction through human waste (medication, farming), animals, and the pharmaceutical industry.[73] The contribution of the pharmaceutical industry is so significant that parallels can be drawn between countries with highest rate of increasing antibiotic resistance and countries with largest footprint of pharmaceutical industry. China, which contributes to nearly 90 per cent of the world's active pharmaceutical ingredient (API) manufacturing, has seen a 22 per cent increase in rate of antimicrobial resistance in six years, compared to a 6 per cent increase in the United States. [74]Along with antibiotic waste, resistant bacteria follow, thus introducing antibiotic-resistant bacteria into the environment. Already in 2011, mapping of sewage and water supply samples in New Delhi showed widespread and uncontrolled infection as indicated by the presence of NDM-1-positive enteric bacteria (New Delhi metallo-beta-lactamase 1).[75]
As bacteria replicate quickly, the resistant bacteria that enter water bodies through wastewater replicate their resistance genes as they continue to divide. In addition, bacteria carrying resistance genes have the ability to spread those genes to other species via horizontal gene transfer. Therefore, even if the specific antibiotic is no longer introduced into the environment, antibiotic-resistance genes will persist through the bacteria that have since replicated without continuous exposure.[73] Antibiotic resistance is widespread in marine vertebrates, and they may be important reservoirs of antibiotic-resistant bacteria in the marine environment.[76]
Prevention
Mission Critical: Preventing Antibiotic Resistance (CDC report, 2014)
There have been increasing public calls for global collective action to address the threat, including a proposal for international treaty on antimicrobial resistance. Further detail and attention is still needed in order to recognize and measure trends in resistance on the international level; the idea of a global tracking system has been suggested but implementation has yet to occur. A system of this nature would provide insight to areas of high resistance as well as information necessary for evaluation of programs and other changes made to fight or reverse antibiotic resistance.
Five important strategies needed for minimising antibiotic resistance are as follows:[77]
- Antibiotic stewardship to maintain the value of existing and future antibiotics
- The timing of prescription to use the effective antibiotics sooner rather than later
- To develop and approve ten new antibiotics by 2020
- Development of a molecular method for detecting antibiotic resistance genes
- To avoid the delay in distribution of US$2 billion global antibiotic resistance innovation fund.
Duration of antibiotics
Antibiotic treatment duration should be based on the infection and other health problems a person may have.[9] For many infections once a person has improved there is little evidence that stopping treatment causes more resistance.[9] Some therefore feel that stopping early may be reasonable in some cases.[9] Other infections, however, do require long courses regardless of whether a person feels better.[9]Monitoring and mapping
There are multiple national and international monitoring programs for drug-resistant threats, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant S. aureus (VRSA), extended spectrum beta-lactamase (ESBL), vancomycin-resistant Enterococcus (VRE), multidrug-resistant A. baumannii (MRAB).[78]ResistanceOpen is an online global map of antimicrobial resistance developed by HealthMap which displays aggregated data on antimicrobial resistance from publicly available and user submitted data.[79][80] The website can display data for a 25-mile radius from a location. Users may submit data from antibiograms for individual hospitals or laboratories. European data is from the EARS-Net (European Antimicrobial Resistance Surveillance Network), part of the ECDC.
ResistanceMap is a website by the Center for Disease Dynamics, Economics & Policy and provides data on antimicrobial resistance on a global level.[81]
Limiting antibiotic use
Antibiotic stewardship programmes appear useful in reducing rates of antibiotic resistance.[82]Excessive antibiotic use has become one of the top contributors to the development of antibiotic resistance. Since the beginning of the antibiotic era, antibiotics have been used to treat a wide range of disease.[83] Overuse of antibiotics has become the primary cause of rising levels of antibiotic resistance. The main problem is that doctors are willing to prescribe antibiotics to ill-informed individuals who believe that antibiotics can cure nearly all illnesses, including viral infections like the common cold. In an analysis of drug prescriptions, 36% of individuals with a cold or an upper respiratory infection (both viral in origin) were given prescriptions for antibiotics.[84] These prescriptions accomplished nothing other than increasing the risk of further evolution of antibiotic resistant bacteria.
At the hospital level
Antimicrobial stewardship teams in hospitals are encouraging optimal use of antimicrobials.[85] The goals of antimicrobial stewardship are to help practitioners pick the right drug at the right dose and duration of therapy while preventing misuse and minimizing the development of resistance. Stewardship may reduce the length of stay by an average of slightly over 1 day while not increasing the risk of death.[86]At the level of GP
Given the volume of care provided in primary care (General Practice), recent strategies have focused on reducing unnecessary antibiotic prescribing in this setting. Simple interventions, such as written information explaining the futility of antibiotics for common infections such as upper respiratory tract infections, have been shown to reduce antibiotic prescribing.[87]The prescriber should closely adhere to the five rights of drug administration: the right patient, the right drug, the right dose, the right route, and the right time.[88]
Cultures should be taken before treatment when indicated and treatment potentially changed based on the susceptibility report.[10][89]
About a third of antibiotic prescriptions written in outpatient settings in the United States were not appropriate in 2010 and 2011. Doctors in the U.S. wrote 506 annual antibiotic scripts for every 1,000 people, with 353 being medically necessary.[90]
Health workers and pharmacists can help tackle resistance by: enhancing infection prevention and control; only prescribing and dispensing antibiotics when they are truly needed; prescribing and dispensing the right antibiotic(s) to treat the illness.[20]
At the individual level
People can help tackle resistance by using antibiotics only when prescribed by a doctor; completing the full prescription, even if they feel better; never sharing antibiotics with others or using leftover prescriptions.[20]Country examples
- The Netherlands has the lowest rate of antibiotic prescribing in the OECD, at a rate of 11.4 defined daily doses (DDD) per 1,000 people per day in 2011.
- Germany and Sweden also have lower prescribing rates, with Sweden's rate having been declining since 2007.
- Greece, France and Belgium have high prescribing rates of more than 28 DDD.[91]
Water, sanitation, hygiene
Infectious disease control through improved water, sanitation and hygiene (WASH) infrastructure needs to be placed at the center of the antimicrobial resistance (AMR) agenda. The spread of infectious diseases caused by inadequate WASH standards is a major driver of antibiotic demand in developing countries.[34] Growing usage of antibiotics together with persistent infectious disease levels have led to a dangerous cycle in which reliance on antimicrobials increases while the efficacy of drugs diminishes.[34] The proper use of infrastructure for water, sanitation and hygiene (WASH) can result in a 47–72 percent decrease of diarrhea cases treated with antibiotics depending on the type of intervention and its effectiveness.[34] A reduction of the diarrhea disease burden through improved infrastructure would result in large decreases in the number of diarrhea cases treated with antibiotics. This was estimated as ranging from 5 million in Brazil to up to 590 million in India by the year 2030.[34] The strong link between increased consumption and resistance indicates that this will directly mitigate the accelerating spread of AMR.[34] Sanitation and water for all by 2030 is Goal Number 6 of the Sustainable Development Goals.An increase in hand washing compliance by hospital staff results in decreased rates of resistant organisms.[92]
Management in animal use
Europe
In 1997, European Union health ministers voted to ban avoparcin and four additional antibiotics used to promote animal growth in 1999.[93] In 2006 a ban on the use of antibiotics in European feed, with the exception of two antibiotics in poultry feeds, became effective.[94] In Scandinavia, there is evidence that the ban has led to a lower prevalence of antibiotic resistance in (nonhazardous) animal bacterial populations.[95] As of 2004, several European countries established a decline of antimicrobial resistance in humans through limiting the usage antimicrobials in agriculture and food industries without jeopardizing animal health or economic cost.[96]United States
The United States Department of Agriculture (USDA) and the Food and Drug Administration (FDA) collect data on antibiotic use in humans and in a more limited fashion in animals.[97] The FDA first determined in 1977 that there is evidence of emergence of antibiotic-resistant bacterial strains in livestock. The long-established practice of permitting OTC sales of antibiotics (including penicillin and other drugs) to lay animal owners for administration to their own animals nonetheless continued in all states. In 2000, the FDA announced their intention to revoke approval of fluoroquinolone use in poultry production because of substantial evidence linking it to the emergence of fluoroquinolone-resistant Campylobacter infections in humans. Legal challenges from the food animal and pharmaceutical industries delayed the final decision to do so until 2006.[98] Fluroquinolones have been banned from extra-label use in food animals in the USA since 2007. However, they remain widely used in companion and exotic animals.Global action plans and awareness
The increasing interconnectedness of the world and the fact that new classes of antibiotics have not been developed and approved for more than 25 years highlight the extent to which antimicrobial resistance is a global health challenge.[99] A global action plan to tackle the growing problem of resistance to antibiotics and other antimicrobial medicines was endorsed at the Sixty-eighth World Health Assembly in May 2015.[100] One of the key objectives of the plan is to improve awareness and understanding of antimicrobial resistance through effective communication, education and training. This global action plan developed by the World Health Organization was created to combat the issue of antimicrobial resistance and was guided by the advice of countries and key stakeholders. The WHO's global action plan is composed of five key objectives that can be targeted through different means, and represents countries coming together to solve a major problem that can have future health consequences.[100]- React based in Sweden has produced informative material on AMR for the general public.[101]
Antibiotic Awareness Week
The World Health Organization has promoted the first World Antibiotic Awareness Week running from 16–22 November 2015. The aim of the week is to increase global awareness of antibiotic resistance. It also wants to promote the correct usage of antibiotics across all fields in order to prevent further instances of antibiotic resistance.[104]World Antibiotic Awareness Week has been held every November since 2015. For 2017, the Food and Agriculture Organization of the United Nations (FAO), the World Health Organization (WHO) and the World Organisation for Animal Health (OIE) are together calling for responsible use of antibiotics in humans and animals to reduce the emergence of antibiotic resistance.[105]
Mechanisms and organisms
Fundamentals
Diagram depicting antibiotic resistance through alteration of the
antibiotic's target site, modeled after MRSA's resistance to penicillin.
Beta-lactam antibiotics permanently inactivate PBP enzymes, which are essential for bacterial life, by permanently binding to their active sites. MRSA, however, expresses a PBP that does not allow the antibiotic into its active site.
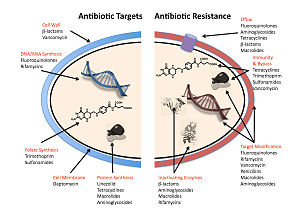
The four main mechanisms by which microorganisms exhibit resistance to antimicrobials are:
- Drug inactivation or modification: for example, enzymatic deactivation of penicillin G in some penicillin-resistant bacteria through the production of β-lactamases. The emergence of carbapenem-resistant Gram-negative pathogens poses a serious threat to public health worldwide. Klebsiella pneumoniae carbapenemases (KPCs) and carbapenemases of the oxacillinase-48 (OXA-48) type have been reported worldwide. New Delhi metallo-β-lactamase (NDM) carbapenemases were originally identified in Sweden in 2008 and have spread worldwide rapidly.[106] Most commonly, the protective enzymes produced by the bacterial cell will add an acetyl or phosphate group to a specific site on the antibiotic, which will reduce its ability to bind to the bacterial ribosomes and disrupt protein synthesis.[107]
- Alteration of target- or binding site: for example, alteration of PBP—the binding target site of penicillins—in MRSA and other penicillin-resistant bacteria. Another protective mechanism found among bacterial species is ribosomal protection proteins. These proteins protect the bacterial cell from antibiotics that target the cell’s ribosomes to inhibit protein synthesis. The mechanism involves the binding of the ribosomal protection proteins to the ribosomes of the bacterial cell, which in turn changes its conformational shape. This allows the ribosomes to continue synthesizing proteins essential to the cell while preventing antibiotics from binding to the ribosome to inhibit protein synthesis.[citation needed]
- Alteration of metabolic pathway: for example, some sulfonamide-resistant bacteria do not require para-aminobenzoic acid (PABA), an important precursor for the synthesis of folic acid and nucleic acids in bacteria inhibited by sulfonamides, instead, like mammalian cells, they turn to using preformed folic acid.[citation needed]
- Reduced drug accumulation: by decreasing drug permeability or increasing active efflux (pumping out) of the drugs across the cell surface[108] These pumps within the cellular membrane of certain bacterial species are used to pump antibiotics out of the cell before they are able to do any damage. They are often activated by a specific substrate associated with an antibiotic.[109] as in fluoroquinolone resistance.[110]
A number of mechanisms used by common antibiotics to deal with bacteria and ways by which bacteria become resistant to them.
Antibiotic resistance can be a result of horizontal gene transfer,[111] and also of unlinked point mutations in the pathogen genome at a rate of about 1 in 108 per chromosomal replication. Mutations are rare but the fact that bacteria reproduce at such a high rate allows for the effect to be significant. A mutation may produce a change in the binding site of the antibiotic, which may allow the site to continue proper functioning in the presence of the antibiotic or prevent the binding of the antibiotic to the site altogether.[112]
Antibiotic action against a pathogen can be seen as an environmental pressure. Those bacteria with a mutation that allows them to survive will reproduce, pass the trait to their offspring, which leads to the microevolution of a fully resistant colony. Chromosomal mutations providing antibiotic resistance benefit the bacteria but also confer a cost of fitness. For example, a ribosomal mutation may protect a bacterial cell by changing the binding site of an antibiotic but will also slow protein synthesis.[107] manifesting, in slower growth rate.[113]
In Gram-negative bacteria, plasmid-mediated resistance genes produce proteins that can bind to DNA gyrase, protecting it from the action of quinolones. Finally, mutations at key sites in DNA gyrase or topoisomerase IV can decrease their binding affinity to quinolones, decreasing the drug's effectiveness.[114]
Bacteria
Klebsiella pneumoniae, the bacterium in which NDM-1 was first identified.
Bacteria can develop antibiotic resistance. Recent findings show no necessity of large populations of bacteria for the appearance of antibiotic resistance. Small populations of E. coli in an antibiotic gradient can become resistant. Any heterogeneous environment with respect to nutrient and antibiotic gradients may facilitate antibiotic resistance in small bacterial populations. Researchers hypothesize that the mechanism of resistance development is based on four SNP mutations in the genome of E. coli produced by the gradient of antibiotic.[citation needed]
Antibiotic resistance can be introduced artificially into a microorganism through laboratory protocols, sometimes used as a selectable marker to examine the mechanisms of gene transfer or to identify individuals that absorbed a piece of DNA that included the resistance gene and another gene of interest.[115]
New Delhi metallo-beta-lactamase 1 (NDM-1)[116] is an enzyme that makes bacteria resistant to a broad range of beta-lactam antibiotics. The most common bacteria that make this enzyme are gram-negative such as Escherichia coli and Klebsiella pneumoniae, but the gene for NDM-1 can spread from one strain of bacteria to another by horizontal gene transfer.[117]
Viruses
Specific antiviral drugs are used to treat some viral infections. These drugs prevent viruses from reproducing by inhibiting essential stages of the virus's replication cycle in infected cells. Antivirals are used to treat HIV, hepatitis B, hepatitis C, influenza, herpes viruses including varicella zoster virus, cytomegalovirus and Epstein-Barr virus. With each virus, some strains have become resistant to the administered drugs.[118]Resistance to HIV antivirals is problematic, and even multi-drug resistant strains have evolved.[119] Resistant strains of the HIV virus emerge rapidly if only one antiviral drug is used.[120] Using three or more drugs together has helped to control this problem, but new drugs are needed because of the continuing emergence of drug-resistant HIV strains.[121]
Fungi
Infections by fungi are a cause of high morbidity and mortality in immunocompromised persons, such as those with HIV/AIDS, tuberculosis or receiving chemotherapy.[122] The fungi candida, Cryptococcus neoformans and Aspergillus fumigatus cause most of these infections and antifungal resistance occurs in all of them.[123] Multidrug resistance in fungi is increasing because of the widespread use of antifungal drugs to treat infections in immunocompromised individuals.[124]Of particular note, Fluconazole-resistant Candida species have been highlighted as a growing problem by the CDC.[78] More than 20 Candida species of Candida can cause Candidiasis infection, the most common of which is Candida albicans. Candida yeasts normally inhabit the skin and mucous membranes without causing infection. However, overgrowth of Candida can lead to Candidiasis. Some Candida strains are becoming resistant to first-line and second-line antifungal agents such as azoles and echinocandins.[78]
Parasites
The protozoan parasites that cause the diseases malaria, trypanosomiasis, toxoplasmosis, cryptosporidiosis and leishmaniasis are important human pathogens.[125]Malarial parasites that are resistant to the drugs that are currently available to infections are common and this has led to increased efforts to develop new drugs.[126] Resistance to recently developed drugs such as artemisinin has also been reported. The problem of drug resistance in malaria has driven efforts to develop vaccines.[127]
Trypanosomes are parasitic protozoa that cause African trypanosomiasis and Chagas disease (American trypanosomiasis).[128][129] There are no vaccines to prevent these infections so drugs such as pentamidine and suramin, benznidazole and nifurtimox and used to treat infections. These drugs are effective but infections caused by resistant parasites have been reported.[125]
Leishmaniasis is caused by protozoa and is an important public health problem worldwide, especially in sub-tropical and tropical countries. Drug resistance has "become a major concern".[130]
History
The discovery of penicillin in 1928 and other antibiotics in the 20th century proved to be a significant medical achievement, saving millions of lives and significantly reducing the burden of infectious diseases.[131] The 1950s to 1970s represented the golden age of antibiotic discovery, where countless new classes of antibiotics were discovered to treat previously incurable diseases such as tuberculosis and syphilis.[132] However, since that time the discovery of new classes of antibiotics has been almost nonexistent, and represents a situation that is especially problematic considering the resiliency of bacteria shown over time and the continued misuse and overuse of antibiotics in treatment.[133]The phenomenon of antimicrobial resistance caused by overuse of antibiotics was predicted already by Alexander Fleming who said "The time may come when penicillin can be bought by anyone in the shops. Then there is the danger that the ignorant man may easily under-dose himself and by exposing his microbes to nonlethal quantities of the drug make them resistant."[134][135] Without the creation of new and stronger antibiotics an era where common infections and minor injuries can kill this century, with complex procedures such as surgery and chemotherapy becoming too risky, is a very real possibility.[136] Antimicrobial resistance threatens the world as we know it, and can lead to epidemics of enormous proportions if preventive actions are not taken. In this day and age current antimicrobial resistance leads to longer hospital stays, higher medical costs, and increased mortality.[137]
Society and culture
For the fiscal year 2016 budget, President Obama has suggested to nearly double the amount of federal funding to "combat and prevent" antibiotic resistance to more than $1.2 billion.[138] Many international funding agencies like USAID, DFID, SIDA and Bill & Melinda Gates Foundation has pledged money for developing strategies to counter antimicrobial resistance.Since the mid-1980s pharmaceutical companies have invested in medications for cancer or chronic disease that have greater potential to make money and have "de-emphasized or dropped development of antibiotics".[139] On January 20, 2016 at the World Economic Forum in Davos, Switzerland, more than "80 pharmaceutical and diagnostic companies" from around the world called for 'transformational commercial models' at a global level to spur research and development on antibiotics and on the "enhanced use of diagnostic tests that can rapidly identify the infecting organism".[139]
Legal frameworks
Some global health scholars have argued that a global, legal framework is needed to prevent and control antimicrobial resistance.[140][141][17][142] For instance, binding global policies could be used to create antimicrobial use standards, regulate antibiotic marketing, and strengthen global surveillance systems.[17][140] Ensuring compliance of involved parties is a challenge.[17] Global antimicrobial resistance policies could take lessons from the environmental sector by adopting strategies that have made international environmental agreements successful in the past such as: sanctions for non-compliance, assistance for implementation, majority vote decision-making rules, an independent scientific panel, and specific commitments.[143]U.S.
On March 27, 2015, the White House released a comprehensive plan to address the increasing need for agencies to combat the rise of antibiotic-resistant bacteria. The Task Force for Combating Antibiotic-Resistant Bacteria developed The National Action Plan for Combating Antibiotic-Resistant Bacteria with the intent of providing a roadmap to guide the US in the antibiotic resistance challenge and with hopes of saving many lives. This plan outlines steps taken by the Federal government over the next five years needed in order to prevent and contain outbreaks of antibiotic-resistant infections; maintain the efficacy of antibiotics already on the market; and to help to develop future diagnostics, antibiotics, and vaccines.[144]The Action Plan was developed around five goals with focuses on strengthening health care, public health veterinary medicine, agriculture, food safety and research, and manufacturing. These goals, as listed by the White House, are as follows:
- Slow the Emergence of Resistant Bacteria and Prevent the Spread of Resistant Infections
- Strengthen National One-Health Surveillance Efforts to Combat Resistance
- Advance Development and use of Rapid and Innovative Diagnostic Tests for Identification and Characterization of Resistant Bacteria
- Accelerate Basic and Applied Research and Development for New Antibiotics, Other Therapeutics, and Vaccines
- Improve International Collaboration and Capacities for Antibiotic Resistance Prevention, Surveillance, Control and Antibiotic Research and Development
- Establishment of antimicrobial programs within acute care hospital settings
- Reduction of inappropriate antibiotic prescription and use by at least 50% in outpatient settings and 20% inpatient settings
- Establishment of State Antibiotic Resistance (AR) Prevention Programs in all 50 states
- Elimination of the use of medically important antibiotics for growth promotion in food-producing animals.
Policies
According to WHO policymakers can help tackle resistance by strengthening resistance tracking and laboratory capacity; regulating and promoting appropriate use of medicines.[20] Policymakers and industry can help tackle resistance by: fostering innovation and research and development of new tools; promoting cooperation and information sharing among all stakeholders.[20]Further research
It is unclear if rapid viral testing affects antibiotic use in children.[145]Vaccines
Microorganisms do not develop resistance to vaccines because a vaccine enhances the body's immune system, whereas an antibiotic operates separately from the body's normal defenses. Furthermore, if the use of vaccines increase, there is evidence that antibiotic resistant strains of pathogens will decrease; the need for antibiotics will naturally decrease as vaccines prevent infection before it occurs.[146] However, new strains that escape immunity induced by vaccines may evolve; for example, an updated influenza vaccine is needed each year.While theoretically promising, antistaphylococcal vaccines have shown limited efficacy, because of immunological variation between Staphylococcus species, and the limited duration of effectiveness of the antibodies produced. Development and testing of more effective vaccines is underway.[147]
Alternating therapy
Alternating therapy is a proposed method in which two or three antibiotics are taken in a rotation versus taking just one antibiotic such that bacteria resistant to one antibiotic are killed when the next antibiotic is taken. Studies have found that this method reduces the rate at which antibiotic resistant bacteria emerge in vitro relative to a single drug for the entire duration.[148]Studies have found that bacteria that evolve antibiotic resistance towards one group of antibiotic may become more sensitive others.[149] This phenomona can be utilized to select against resistant bacteria using an approach termed collateral sensitivity cycling,[150] which has recently been found to be relevant in developing treatment strategies for chronic infections caused by Pseudomonas aeruginosa.[151]
Development of new drugs
Since the discovery of antibiotics, research and development (R&D) efforts have provided new drugs in time to treat bacteria that became resistant to older antibiotics, but in the 2000s there has been concern that development has slowed enough that seriously ill people may run out of treatment options.[152] Another concern is that doctors may become reluctant to perform routine surgeries because of the increased risk of harmful infection.[153] Backup treatments can have serious side-effects; for example, treatment of multi-drug-resistant tuberculosis can cause deafness or psychological disability.[154] The potential crisis at hand is the result of a marked decrease in industry R&D.[155] Poor financial investment in antibiotic research has exacerbated the situation.[156][155] The pharmaceutical industry has little incentive to invest in antibiotics because of the high risk and because the potential financial returns are less likely to cover the cost of development than for other pharmaceuticals.[157] In 2011, Pfizer, one of the last major pharmaceutical companies developing new antibiotics, shut down its primary research effort, citing poor shareholder returns relative to drugs for chronic illnesses.[158] However, small and medium-sized pharmaceutical companies are still active in antibiotic drug research.In the United States, drug companies and the administration of President Barack Obama have been proposing changing the standards by which the FDA approves antibiotics targeted at resistant organisms.[153][159] On 12 December 2013, the Antibiotic Development to Advance Patient Treatment (ADAPT) Act of 2013 was introduced in the U.S. Congress. The ADAPT Act aims to fast-track the drug development in order to combat the growing public health threat of 'superbugs'. Under this Act, the FDA can approve antibiotics and antifungals needed for life-threatening infections based on data from smaller clinical trials. The Centers for Disease Control and Prevention (CDC) will reinforce the monitoring of the use of antibiotics that treat serious and life-threatening infections and the emerging resistance, and make the data publicly available. The FDA antibiotics labeling process, 'Susceptibility Test Interpretive Criteria for Microbial Organisms' or 'breakpoints' is also streamlined to allow the most up-to-date and cutting-edge data available to healthcare professionals under the new Act.[160][161]
On 18 September 2014 Obama signed an executive order[162] to implement the recommendations proposed in a report[163] by the President's Council of Advisors on Science and Technology (PCAST) which outlines strategies to stream-line clinical trials and speed up the R&D of new antibiotics. Among the proposals:
- Create a 'robust, standing national clinical trials network for antibiotic testing' which will promptly enroll patients once identified to be suffering from dangerous bacterial infections. The network will allow testing multiple new agents from different companies simultaneously for their safety and efficacy.
- Establish a 'Special Medical Use (SMU)' pathway for FDA to approve new antimicrobial agents for use in limited patient populations, shorten the approval timeline for new drug so patients with severe infections could benefit as quickly as possible.
- Provide economic incentives, especially for development of new classes of antibiotics, to offset the steep R&D costs which drive away the industry to develop antibiotics.
The U.S. National Institutes of Health plans to fund a new research network on the issue up to $62 million from 2013 to 2019.[165] Using authority created by the Pandemic and All Hazards Preparedness Act of 2006, the Biomedical Advanced Research and Development Authority in the U.S. Department of Health and Human Services announced that it will spend between $40 million and $200 million in funding for R&D on new antibiotic drugs under development by GlaxoSmithKline.[166]
Phage therapy
Phage therapy is the therapeutic use of bacteriophages to treat pathogenic bacterial infections.[167] Phage therapy has many potential applications in human medicine as well as dentistry, veterinary science, and agriculture.[168]Phage therapy relies on the use of naturally-occurring bacteriophages to infect and lyse bacteria at the site of infection in a host. Due to current advances in genetics and biotechnology these bacteriophages can possibly be manufactured to treat specific infections.[169] Phages can be bioengineered to target multidrug-resistant bacterial infections, and their use involves the added benefit of preventing the elimination of beneficial bacteria in the human body. [169] Phages destroy bacterial cell walls and membrane through the use of lytic proteins which kill bacteria by making many holes from the inside out.[170] Bacteriophages can even possess the ability to digest the biofilm that many bacteria develop that protect them from antibiotics in order to effectively infect and kill bacteria. Bioengineering can play a role in creating successful bacteriophages.[170]
Bacteriophages are much more specific than antibiotics. They are typically harmless not only to the host organism, but also to other beneficial bacteria, such as the gut flora, reducing the chances of opportunistic infections.[171]
Bacteriophages are used against antibiotic resistant bacteria in Georgia (George Eliava Institute) and in one institute in Wrocław, Poland.[172][173]