| Cytochrome C Oxidase | |
|---|---|
| |
| Identifiers | |
| EC number | 1.9.3.1 |
| CAS number | 9001-16-5 |
| Databases | |
| IntEnz | IntEnz view |
| BRENDA | BRENDA entry |
| ExPASy | NiceZyme view |
| KEGG | KEGG entry |
| MetaCyc | metabolic pathway |
| PRIAM | profile |
| PDB structures | RCSB PDB PDBe PDBsum |
| Gene Ontology | AmiGO / QuickGO |
| Cytochrome c oxidase | |
|---|---|

| |
| Identifiers | |
| Symbol | Cytochrome c oxidase |
| OPM superfamily | 4 |
| OPM protein | 2dyr |
| Membranome | 257 |
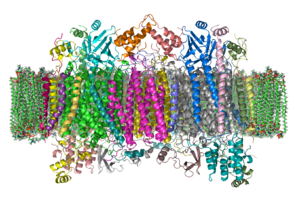
The enzyme cytochrome c oxidase or Complex IV, EC 1.9.3.1 is a large transmembrane protein complex found in bacteria, archaea, and in eukaryotes in their mitochondria.
It is the last enzyme in the respiratory electron transport chain of cells located in the membrane. It receives an electron from each of four cytochrome c molecules, and transfers them to one dioxygen molecule, converting the molecular oxygen
to two molecules of water. In this process it binds four protons from
the inner aqueous phase to make two water molecules, and translocates
another four protons across the membrane, increasing the transmembrane
difference of proton electrochemical potential which the ATP synthase then uses to synthesize ATP.
Structure
The complex
The complex is a large integral membrane protein composed of several metal prosthetic sites and 14
protein subunits in mammals. In mammals, eleven subunits are nuclear in
origin, and three are synthesized in the mitochondria. The complex
contains two hemes, a cytochrome a and cytochrome a3, and two copper centers, the CuA and CuB centers. In fact, the cytochrome a3 and CuB form a binuclear center that is the site of oxygen reduction. Cytochrome c, which is reduced by the preceding component of the respiratory chain (cytochrome bc1 complex, complex III), docks near the CuA binuclear center and passes an electron to it, being oxidized back to cytochrome c containing Fe3+. The reduced CuA binuclear center now passes an electron on to cytochrome a, which in turn passes an electron on to the cytochrome a3-CuB binuclear center. The two metal ions in this binuclear center are 4.5 Å apart and coordinate a hydroxide ion in the fully oxidized state.
Crystallographic studies
of cytochrome c oxidase show an unusual post-translational
modification, linking C6 of Tyr(244) and the ε-N of His(240) (bovine
enzyme numbering). It plays a vital role in enabling the cytochrome a3- CuB binuclear center to accept four electrons in reducing molecular oxygen to water. The mechanism of reduction was formerly thought to involve a peroxide intermediate, which was believed to lead to superoxide
production. However, the currently accepted mechanism involves a rapid
four-electron reduction involving immediate oxygen-oxygen bond cleavage,
avoiding any intermediate likely to form superoxide.
The conserved subunits
| No. | Subunit name | Human protein | Protein description from UniProt | Pfam family with Human protein |
|---|---|---|---|---|
| 1 | Cox1 | COX1_HUMAN | Cytochrome c oxidase subunit 1 | Pfam PF00115 |
| 2 | Cox2 | COX2_HUMAN | Cytochrome c oxidase subunit 2 | Pfam PF02790, Pfam PF00116 |
| 3 | Cox3 | COX3_HUMAN | Cytochrome c oxidase subunit 3 | Pfam PF00510 |
| 4 | Cox4i1 | COX41_HUMAN | Cytochrome c oxidase subunit 4 isoform 1, mitochondrial | Pfam PF02936 |
| 5 | Cox4a2 | COX42_HUMAN | Cytochrome c oxidase subunit 4 isoform 2, mitochondrial | Pfam PF02936 |
| 6 | Cox5a | COX5A_HUMAN | Cytochrome c oxidase subunit 5A, mitochondrial | Pfam PF02284 |
| 7 | Cox5b | COX5B_HUMAN | Cytochrome c oxidase subunit 5B, mitochondrial | Pfam PF01215 |
| 8 | Cox6a1 | CX6A1_HUMAN | Cytochrome c oxidase subunit 6A1, mitochondrial | Pfam PF02046 |
| 9 | Cox6a2 | CX6A2_HUMAN | Cytochrome c oxidase subunit 6A2, mitochondrial | Pfam PF02046 |
| 10 | Cox6b1 | CX6B1_HUMAN | Cytochrome c oxidase subunit 6B1 | Pfam PF02297 |
| 11 | Cox6b2 | CX6B2_HUMAN | Cytochrome c oxidase subunit 6B2 | Pfam PF02297 |
| 12 | Cox6c | COX6C_HUMAN | Cytochrome c oxidase subunit 6C | Pfam PF02937 |
| 13 | Cox7a1 | CX7A1_HUMAN | Cytochrome c oxidase subunit 7A1, mitochondrial | Pfam PF02238 |
| 14 | Cox7a2 | CX7A2_HUMAN | Cytochrome c oxidase subunit 7A2, mitochondrial | Pfam PF02238 |
| 15 | Cox7a3 | COX7S_HUMAN | Putative cytochrome c oxidase subunit 7A3, mitochondrial | Pfam PF02238 |
| 16 | Cox7b | COX7B_HUMAN | Cytochrome c oxidase subunit 7B, mitochondrial | Pfam PF05392 |
| 17 | Cox7c | COX7C_HUMAN | Cytochrome c oxidase subunit 7C, mitochondrial | Pfam PF02935 |
| 18 | Cox7r | COX7R_HUMAN | Cytochrome c oxidase subunit 7A-related protein, mitochondrial | Pfam PF02238 |
| 19 | Cox8a | COX8A_HUMAN | Cytochrome c oxidase subunit 8A, mitochondrial P | Pfam PF02285 |
| 20 | Cox8c | COX8C_HUMAN | Cytochrome c oxidase subunit 8C, mitochondrial | Pfam PF02285 |
| Assembly subunits | ||||
| 1 | Coa1 | COA1_HUMAN | Cytochrome c oxidase assembly factor 1 homolog | Pfam PF08695 |
| 2 | Coa3 | COA3_HUMAN | Cytochrome c oxidase assembly factor 3 homolog, mitochondrial | Pfam PF09813 |
| 3 | Coa4 | COA4_HUMAN | Cytochrome c oxidase assembly factor 4 homolog, mitochondrial | Pfam PF06747 |
| 4 | Coa5 | COA5_HUMAN | Cytochrome c oxidase assembly factor 5 | Pfam PF10203 |
| 5 | Coa6 | COA6_HUMAN | Cytochrome c oxidase assembly factor 6 homolog | Pfam PF02297 |
| 6 | Coa7 | COA7_HUMAN | Cytochrome c oxidase assembly factor 7, | Pfam PF08238 |
| 7 | Cox11 | COX11_HUMAN | Cytochrome c oxidase assembly protein COX11 mitochondrial | Pfam PF04442 |
| 8 | Cox14 | COX14_HUMAN | Cytochrome c oxidase assembly protein | Pfam PF14880 |
| 9 | Cox15 | COX15_HUMAN | Cytochrome c oxidase assembly protein COX15 homolog | Pfam PF02628 |
| 10 | Cox16 | COX16_HUMAN | Cytochrome c oxidase assembly protein COX16 homolog mitochondrial | Pfam PF14138 |
| 11 | Cox17 | COX17_HUMAN | Cytochrome c oxidase copper chaperone | Pfam PF05051 |
| 12 | Cox18[10] | COX18_HUMAN | Mitochondrial inner membrane protein (Cytochrome c oxidase assembly protein 18) | Pfam PF02096 |
| 13 | Cox19 | COX19_HUMAN | Cytochrome c oxidase assembly protein | Pfam PF06747 |
| 14 | Cox20 | COX20_HUMAN | Cytochrome c oxidase protein 20 homolog | Pfam PF12597 |
Assembly
COX assembly in yeast
is a complex process that is not entirely understood due to the rapid
and irreversible aggregation of hydrophobic subunits that form the
holoenzyme complex, as well as aggregation of mutant subunits with
exposed hydrophobic patches.
COX subunits are encoded in both the nuclear and mitochondrial genomes.
The three subunits that form the COX catalytic core are encoded in the
mitochondrial genome.
Hemes and cofactors are inserted into subunits I & II. The
two heme molecules reside in subunit I, helping with transport to
subunit II where two copper molecules aid with the continued transfer of
electrons.
Subunits I and IV initiate assembly. Different subunits may associate
to form sub-complex intermediates that later bind to other subunits to
form the COX complex. In post-assembly modifications, COX will form a homodimer. This is required for activity. Both dimers are connected by a cardiolipin molecule,
which has been found to play a key role in stabilization of the
holoenzyme complex. The dissociation of subunits VIIa and III in
conjunction with the removal of cardiolipin results in total loss of
enzyme activity.
Subunits encoded in the nuclear genome are known to play a role in
enzyme dimerization and stability. Mutations to these subunits eliminate
COX function.
Assembly is known to occur in at least three distinct
rate-determining steps. The products of these steps have been found,
though specific subunit compositions have not been determined.
Synthesis and assembly of COX subunits I, II, and III are
facilitated by translational activators, which interact with the 5’
untranslated regions of mitochondrial mRNA transcripts. Translational
activators are encoded in the nucleus. They can operate through either
direct or indirect interaction with other components of translation
machinery, but exact molecular mechanisms are unclear due to
difficulties associated with synthesizing translation machinery
in-vitro.
Though the interactions between subunits I, II, and III encoded within
the mitochondrial genome make a lesser contribution to enzyme stability
than interactions between bigenomic subunits, these subunits are more
conserved, indicating potential unexplored roles for enzyme activity.
Biochemistry
Summary reaction:
4 Fe2+-cytochrome c + 8 H+ + O2 → 4 Fe3+-cytochrome c + 2 H2O + 4 H+
Two electrons are passed from two cytochrome c's, through the CuA and cytochrome a sites to the cytochrome a3- CuB binuclear center, reducing the metals to the Fe2+ form and Cu+. The hydroxide ligand is protonated and lost as water, creating a void between the metals that is filled by O2. The oxygen is rapidly reduced, with two electrons coming from the Fe2+cytochrome a3, which is converted to the ferryl oxo form (Fe4+=O). The oxygen atom close to CuB picks up one electron from Cu+, and a second electron and a proton from the hydroxyl
of Tyr(244), which becomes a tyrosyl radical: The second oxygen is
converted to a hydroxide ion by picking up two electrons and a proton. A
third electron arising from another cytochrome c is passed through the
first two electron carriers to the cytochrome a3- CuB binuclear center, and this electron and two protons convert the tyrosyl radical back to Tyr, and the hydroxide bound to CuB2+ to a water molecule. The fourth electron from another cytochrome c flows through CuA and cytochrome a to the cytochrome a3- CuB binuclear center, reducing the Fe4+=O to Fe3+,
with the oxygen atom picking up a proton simultaneously, regenerating
this oxygen as a hydroxide ion coordinated in the middle of the
cytochrome a3- CuB center as it was at the start
of this cycle. The net process is that four reduced cytochrome c's are
used, along with 4 protons, to reduce O2 to two water molecules.
Inhibition
COX
exists in three conformational states: fully oxidized (pulsed),
partially reduced, and fully reduced. Each inhibitor has a high affinity
to a different state. In the pulsed state, both the heme a3 and the CuB
nuclear centers are oxidized; this is the conformation of the enzyme
that has the highest activity. A two-electron reduction initiates a
conformational change that allows oxygen to bind at the active site to
the partially-reduced enzyme. Four electrons bind to COX to fully reduce
the enzyme. Its fully reduced state, which consists of a reduced Fe2+
at the cytochrome a3 heme group and a reduced CuB+ binuclear center, is
considered the inactive or resting state of the enzyme.
Cyanide, azide, and carbon monoxide all bind to cytochrome c oxidase, inhibiting the protein from functioning and leading to the chemical asphyxiation
of cells. Higher concentrations of molecular oxygen are needed to
compensate for increasing inhibitor concentrations, leading to an
overall reduction in metabolic activity in the cell in the presence of
an inhibitor. Other ligands, such as nitric oxide and hydrogen sulfide,
can also inhibit COX by binding to regulatory sites on the enzyme,
reducing the rate of cellular respiration.
Cyanide is a non-competitive inhibitor for COX,
binding with high affinity to the partially-reduced state of the enzyme
and hindering further reduction of the enzyme. In the pulsed state,
cyanide binds slowly, but with high affinity. The ligand is posited to
electrostatically stabilize both metals at once by positioning itself
between them. A high nitric oxide concentration, such as one added
exogenously to the enzyme, reverses cyanide inhibition of COX.
Nitric oxide can reversibly
bind to either metal ion in the binuclear center to be oxidized to
nitrite. NO and CN will compete with oxygen to bind at the site,
reducing the rate of cellular respiration. Endogenous NO, however, which
is produced at lower levels, augments CN inhibition. Higher levels of
NO, which correlate with the existence of more enzyme in the reduced
state, lead to a greater inhibition of cyanide.
At these basal concentrations, NO inhibition of Complex IV is known to
have beneficial effects, such as increasing oxygen levels in blood
vessel tissues. The inability of the enzyme to reduce oxygen to water
results in a buildup of oxygen, which can diffuse deeper into
surrounding tissues.
NO inhibition of Complex IV has a larger effect at lower oxygen
concentrations, increasing its utility as a vasodilator in tissues of
need.
Hydrogen sulfide
will bind COX in a noncompetitive fashion at a regulatory site on the
enzyme, similar to carbon monoxide. Sulfide has the highest affinity to
either the pulsed or partially reduced states of the enzyme, and is
capable of partially reducing the enzyme at the heme a3 center. It is
unclear whether endogenous H2S levels are sufficient to inhibit the
enzyme. There is no interaction between hydrogen sulfide and the fully
reduced conformation of COX.
Methanol in methylated spirits is converted into formic acid, which also inhibits the same oxidase system. High levels of ATP can allosterically inhibit cytochrome c oxidase, binding from within the mitochondrial matrix.
Extramitochondrial and subcellular localizations
Cytochrome c oxidase has 3 subunits which are encoded by mitochondrial DNA (cytochrome c oxidase subunit I, subunit II, and subunit III). Of these 3 subunits encoded by mitochondrial DNA, two have been identified in extramitochondrial locations. In pancreatic acinar tissue, these subunits were found in zymogen granules. Additionally, in the anterior pituitary, relatively high amounts of these subunits were found in growth hormone secretory granules.
The extramitochondrial function of these cytochrome c oxidase subunits
has not yet been characterized. Besides cytochrome c oxidase subunits,
extramitochondrial localization has also been observed for large numbers
of other mitochondrial proteins.
This raises the possibility about existence of yet unidentified
specific mechanisms for protein translocation from mitochondria to other
cellular destinations.
Genetic defects and disorders
Defects involving genetic mutations altering cytochrome c oxidase (COX) functionality or structure can result in severe, often fatal metabolic disorders.
Such disorders usually manifest in early childhood and affect
predominantly tissues with high energy demands (brain, heart, muscle).
Among the many classified mitochondrial diseases, those involving dysfunctional COX assembly are thought to be the most severe.
The vast majority of COX disorders are linked to mutations in
nuclear-encoded proteins referred to as assembly factors, or assembly
proteins. These assembly factors contribute to COX structure and
functionality, and are involved in several essential processes,
including transcription and translation of mitochondrion-encoded
subunits, processing of preproteins and membrane insertion, and cofactor
biosynthesis and incorporation.
Currently, mutations have been identified in seven COX assembly factors: SURF1, SCO1, SCO2, COX10, COX15, COX20, COA5 and LRPPRC.
Mutations in these proteins can result in altered functionality of
sub-complex assembly, copper transport, or translational regulation.
Each gene mutation is associated with the etiology of a specific
disease, with some having implications in multiple disorders. Disorders
involving dysfunctional COX assembly via gene mutations include Leigh syndrome, cardiomyopathy, leukodystrophy, anemia, and sensorineural deafness.
Histochemistry
The increased reliance of neurons on oxidative phosphorylation for energy
facilitates the use of COX histochemistry in mapping regional brain
metabolism in animals, since it establishes a direct and positive
correlation between enzyme activity and neuronal activity.
This can be seen in the correlation between COX enzyme amount and
activity, which indicates the regulation of COX at the level of gene
expression. COX distribution is inconsistent across different regions of
the animal brain, but its pattern of its distribution is consistent
across animals. This pattern has been observed in the monkey, mouse, and
calf brain. One isozyme of COX has been consistently detected in
histochemical analysis of the brain.
Such brain mapping has been accomplished in spontaneous mutant mice with cerebellar disease such as reeler and a transgenic model of Alzheimer's disease. This technique has also been used to map learning activity in animal brain.

