Nuclear reprocessing technology was developed to chemically separate and recover fissionable plutonium from spent nuclear fuel. Originally, reprocessing was used solely to extract plutonium for producing nuclear weapons. With commercialization of nuclear power, the reprocessed plutonium was recycled back into MOX nuclear fuel for thermal reactors. The reprocessed uranium,
also known as the spent fuel material, can in principle also be re-used
as fuel, but that is only economical when uranium supply is low and
prices are high. A breeder reactor is not restricted to using recycled plutonium and uranium. It can employ all the actinides, closing the nuclear fuel cycle and potentially multiplying the energy extracted from natural uranium by about 60 times.
Reprocessing has been politically controversial because of the potential to contribute to nuclear proliferation, the potential vulnerability to nuclear terrorism,
the political challenges of repository siting (a problem that applies
equally to direct disposal of spent fuel), the environmental risks of
the aqueous and organic waste streams, and because of its high cost
compared to the once-through fuel cycle.
In the United States, the Obama administration stepped back from
President Bush's plans for commercial-scale reprocessing and reverted to
a program focused on reprocessing-related scientific research. Nuclear fuel reprocessing is performed routinely in Europe, Russia and Japan.
Separated components and disposition
The potentially useful components dealt with in nuclear reprocessing comprise specific actinides (plutonium, uranium, and some minor actinides). The lighter elements components include fission products, activation products, and cladding.
History
The first large-scale nuclear reactors were built during World War II. These reactors were designed for the production of plutonium for use in nuclear weapons. The only reprocessing required, therefore, was the extraction of the plutonium (free of fission-product contamination) from the spent natural uranium
fuel. In 1943, several methods were proposed for separating the
relatively small quantity of plutonium from the uranium and fission
products. The first method selected, a precipitation process called the bismuth phosphate process, was developed and tested at the Oak Ridge National Laboratory (ORNL) between 1943 and 1945 to produce quantities of plutonium for evaluation and use in the US weapons programs. ORNL produced the first macroscopic quantities (grams) of separated plutonium with these processes.
The bismuth phosphate process was first operated on a large scale at the Hanford Site,
in the later part of 1944. It was successful for plutonium separation
in the emergency situation existing then, but it had a significant
weakness: the inability to recover uranium.
The first successful solvent extraction process for the recovery
of pure uranium and plutonium was developed at ORNL in 1949. The PUREX process is the current method of extraction. Separation plants were also constructed at Savannah River Site and a smaller plant at West Valley Reprocessing Plant which closed by 1972 because of its inability to meet new regulatory requirements.
Reprocessing of civilian fuel has long been employed at the COGEMA La Hague site in France, the Sellafield site in the United Kingdom, the Mayak Chemical Combine in Russia, and at sites such as the Tokai plant in Japan, the Tarapur plant in India, and briefly at the West Valley Reprocessing Plant in the United States.
In October 1976, concern of nuclear weapons proliferation (especially after India demonstrated nuclear weapons capabilities using reprocessing technology) led President Gerald Ford to issue a Presidential directive to indefinitely suspend the commercial reprocessing and recycling of plutonium in the U.S. On 7 April 1977, President Jimmy Carter banned the reprocessing of commercial reactor spent nuclear fuel. The key issue driving this policy was the risk of nuclear weapons proliferation by diversion of plutonium from the civilian fuel cycle, and to encourage other nations to follow the USA lead.
After that, only countries that already had large investments in
reprocessing infrastructure continued to reprocess spent nuclear fuel.
President Reagan lifted the ban in 1981, but did not provide the
substantial subsidy that would have been necessary to start up
commercial reprocessing.
In March 1999, the U.S. Department of Energy (DOE) reversed its policy and signed a contract with a consortium of Duke Energy, COGEMA, and Stone & Webster (DCS) to design and operate a mixed oxide (MOX) fuel fabrication facility. Site preparation at the Savannah River Site (South Carolina) began in October 2005.
In 2011 the New York Times reported "...11 years after the government
awarded a construction contract, the cost of the project has soared to
nearly $5 billion. The vast concrete and steel structure is a
half-finished hulk, and the government has yet to find a single
customer, despite offers of lucrative subsidies." TVA (currently the
most likely customer) said in April 2011 that it would delay a decision
until it could see how MOX fuel performed in the nuclear accident at Fukushima Daiichi.
Separation technologies
Water and organic solvents
PUREX
PUREX, the current standard method, is an acronym standing for Plutonium and Uranium Recovery by EXtraction. The PUREX process is a liquid-liquid extraction method used to reprocess spent nuclear fuel, to extract uranium and plutonium, independent of each other, from the fission products. This is the most developed and widely used process in the industry at present.
When used on fuel from commercial power reactors the plutonium
extracted typically contains too much Pu-240 to be considered
"weapons-grade" plutonium, ideal for use in a nuclear weapon.
Nevertheless, highly reliable nuclear weapons can be built at all levels
of technical sophistication using reactor-grade plutonium. Moreover, reactors that are capable of refueling frequently can be used to produce weapon-grade plutonium, which can later be recovered using PUREX. Because of this, PUREX chemicals are monitored.
Plutonium Processing
Modifications of PUREX
UREX
The PUREX process can be modified to make a UREX (URanium EXtraction) process which could be used to save space inside high level nuclear waste disposal sites, such as the Yucca Mountain nuclear waste repository, by removing the uranium which makes up the vast majority of the mass and volume of used fuel and recycling it as reprocessed uranium.
The UREX process is a PUREX process which has been modified to
prevent the plutonium from being extracted. This can be done by adding a
plutonium reductant before the first metal extraction step. In the UREX process, ~99.9% of the uranium and greater than 95% of technetium are separated from each other and the other fission products and actinides. The key is the addition of acetohydroxamic acid
(AHA) to the extraction and scrub sections of the process. The addition
of AHA greatly diminishes the extractability of plutonium and neptunium, providing somewhat greater proliferation resistance than with the plutonium extraction stage of the PUREX process.
TRUEX
Adding a
second extraction agent, octyl(phenyl)-N, N-dibutyl carbamoylmethyl
phosphine oxide(CMPO) in combination with tributylphosphate, (TBP), the
PUREX process can be turned into the TRUEX (TRansUranic EXtraction)
process. TRUEX was invented in the USA by Argonne National Laboratory
and is designed to remove the transuranic metals (Am/Cm) from waste. The
idea is that by lowering the alpha activity
of the waste, the majority of the waste can then be disposed of with
greater ease. In common with PUREX this process operates by a solvation mechanism.
DIAMEX
As an alternative to TRUEX, an extraction process using a malondiamide has been devised. The DIAMEX (DIAMide EXtraction) process has the advantage of avoiding the formation of organic waste which contains elements other than carbon, hydrogen, nitrogen, and oxygen. Such an organic waste can be burned without the formation of acidic gases which could contribute to acid rain (although the acidic gases could be recovered by a scrubber). The DIAMEX process is being worked on in Europe by the French CEA. The process is sufficiently mature that an industrial plant could be constructed with the existing knowledge of the process. In common with PUREX this process operates by a solvation mechanism.
SANEX
Selective ActiNide EXtraction. As part of the management of minor actinides it has been proposed that the lanthanides and trivalent minor actinides should be removed from the PUREX raffinate
by a process such as DIAMEX or TRUEX. In order to allow the actinides
such as americium to be either reused in industrial sources or used as
fuel, the lanthanides must be removed. The lanthanides have large
neutron cross sections and hence they would poison a neutron driven
nuclear reaction. To date the extraction system for the SANEX process
has not been defined, but currently several different research groups
are working towards a process. For instance the French CEA is working on a bis-triazinyl pyridine (BTP) based process.
Other systems such as the dithiophosphinic acids are being worked on by some other workers.
UNEX
The UNiversal EXtraction process was developed in Russia and the Czech Republic; it is designed to completely remove the most troublesome radioisotopes (Sr, Cs and minor actinides) from the raffinate remaining after the extraction of uranium and plutonium from used nuclear fuel. The chemistry is based upon the interaction of caesium and strontium with polyethylene glycol) and a cobalt carborane anion (known as chlorinated cobalt dicarbollide). The actinides are extracted by CMPO, and the diluent is a polar aromatic such as nitrobenzene. Other dilents such as meta-nitrobenzotrifluoride and phenyl trifluoromethyl sulfone have been suggested as well.
Electrochemical methods
Obsolete methods
Bismuth phosphate
The bismuth phosphate process
is an obsolete process that adds significant unnecessary material to
the final radioactive waste. The bismuth phosphate process has been
replaced by solvent extraction processes. The bismuth phosphate process
was designed to extract plutonium from aluminium-clad nuclear fuel rods, containing uranium. The fuel was decladded by boiling it in caustic soda. After decladding, the uranium metal was dissolved in nitric acid.
The plutonium at this point is in the +4 oxidation state. It was then precipitated out of the solution by the addition of bismuth nitrate and phosphoric acid to form the bismuth phosphate. The plutonium was coprecipitated with this. The supernatant liquid (containing many of the fission products) was separated from the solid. The precipitate was then dissolved in nitric acid before the addition of an oxidant (such as potassium permanganate) to produce PuO22+. The plutonium was maintained in the +6 oxidation state by addition of a dichromate salt.
The bismuth phosphate was next re-precipitated, leaving the plutonium in solution, and an iron(II) salt (such as ferrous sulfate) was added. The plutonium was again re-precipitated using a bismuth phosphate carrier and a combination of lanthanum salts and fluoride added, forming a solid lanthanum fluoride carrier for the plutonium. Addition of an alkali
produced an oxide. The combined lanthanum plutonium oxide was collected
and extracted with nitric acid to form plutonium nitrate.
Hexone or redox
This is a liquid-liquid extraction process which uses methyl isobutyl ketone as the extractant. The extraction is by a solvation mechanism. This process has the disadvantage of requiring the use of a salting-out reagent (aluminium nitrate)
to increase the nitrate concentration in the aqueous phase to obtain a
reasonable distribution ratio (D value). Also, hexone is degraded by
concentrated nitric acid. This process has been replaced by the PUREX
process.
Pu4+ + 4 NO3− + 2 S → [Pu(NO3)4S2]
Butex, β,β'-dibutyoxydiethyl ether
A
process based on a solvation extraction process using the triether
extractant named above. This process has the disadvantage of requiring
the use of a salting-out reagent (aluminium nitrate)
to increase the nitrate concentration in the aqueous phase to obtain a
reasonable distribution ratio. This process was used at Windscale many years ago. This process has been replaced by PUREX.
Pyroprocessing
Pyroprocessing is a generic term for high-temperature methods. Solvents are molten salts (e.g. LiCl + KCl or LiF + CaF2) and molten metals (e.g. cadmium, bismuth, magnesium) rather than water and organic compounds. Electrorefining, distillation, and solvent-solvent extraction are common steps.
These processes are not currently in significant use worldwide, but they have been researched and developed at Argonne National Laboratory and elsewhere.
Advantages
- The principles behind them are well understood, and no significant technical barriers exist to their adoption.
- Readily applied to high-burnup spent fuel and requires little cooling time, since the operating temperatures are high already.
- Does not use solvents containing hydrogen and carbon, which are neutron moderators creating risk of criticality accidents and can absorb the fission product tritium and the activation product carbon-14 in dilute solutions that cannot be separated later.
- Alternatively, voloxidation can remove 99% of the tritium from used fuel and recover it in the form of a strong solution suitable for use as a supply of tritium.
- More compact than aqueous methods, allowing on-site reprocessing at the reactor site, which avoids transportation of spent fuel and its security issues, instead storing a much smaller volume of fission products on site as high-level waste until decommissioning. For example, the Integral Fast Reactor and Molten Salt Reactor fuel cycles are based on on-site pyroprocessing.
- It can separate many or even all actinides at once and produce highly radioactive fuel which is harder to manipulate for theft or making nuclear weapons. (However, the difficulty has been questioned.) In contrast the PUREX process was designed to separate plutonium only for weapons, and it also leaves the minor actinides (americium and curium) behind, producing waste with more long-lived radioactivity.
- Most of the radioactivity in roughly 102 to 105 years after the use of the nuclear fuel is produced by the actinides, since there are no fission products with half-lives in this range. These actinides can fuel fast reactors, so extracting and reusing (fissioning) them increases energy production per kg of fuel, as well as reducing the long-term radioactivity of the wastes.
Disadvantages
- Reprocessing as a whole is not currently (2005) in favor, and places that do reprocess already have PUREX plants constructed. Consequently, there is little demand for new pyrometalurgical systems, although there could be if the Generation IV reactor programs become reality.
- The used salt from pyroprocessing is less suitable for conversion into glass than the waste materials produced by the PUREX process.
- If the goal is to reduce the longevity of spent nuclear fuel in burner reactors, then better recovery rates of the minor actinides need to be achieved.
Electrolysis
PYRO-A and -B for IFR
These processes were developed by Argonne National Laboratory and used in the Integral Fast Reactor project.
PYRO-A is a means of separating actinides (elements within the actinide family, generally heavier than U-235) from non-actinides. The spent fuel is placed in an anode basket
which is immersed in a molten salt electrolyte. An electric current is
applied, causing the uranium metal (or sometimes oxide, depending on the
spent fuel) to plate out on a solid metal cathode while the other
actinides (and the rare earths) can be absorbed into a liquid cadmium cathode. Many of the fission products (such as caesium, zirconium and strontium) remain in the salt. As alternatives to the molten cadmium electrode it is possible to use a molten bismuth cathode, or a solid aluminium cathode.
As an alternative to electrowinning, the wanted metal can be isolated by using a molten alloy of an electropositive metal and a less reactive metal.
Since the majority of the long term radioactivity,
and volume, of spent fuel comes from actinides, removing the actinides
produces waste that is more compact, and not nearly as dangerous over
the long term. The radioactivity of this waste will then drop to the
level of various naturally occurring minerals and ores within a few
hundred, rather than thousands of, years.
The mixed actinides produced by pyrometallic processing can be used again as nuclear fuel, as they are virtually all either fissile, or fertile, though many of these materials would require a fast breeder reactor in order to be burned efficiently. In a thermal neutron spectrum, the concentrations of several heavy actinides (curium-242 and plutonium-240)
can become quite high, creating fuel that is substantially different
from the usual uranium or mixed uranium-plutonium oxides (MOX) that most
current reactors were designed to use.
Another pyrochemical process, the PYRO-B process, has been developed for the processing and recycling of fuel from a transmuter reactor ( a fast breeder reactor
designed to convert transuranic nuclear waste into fission products ). A
typical transmuter fuel is free from uranium and contains recovered transuranics in an inert matrix such as metallic zirconium. In the PYRO-B processing of such fuel, an electrorefining
step is used to separate the residual transuranic elements from the
fission products and recycle the transuranics to the reactor for
fissioning. Newly generated technetium and iodine are extracted for
incorporation into transmutation targets, and the other fission products
are sent to waste.
Voloxidation
Voloxidation (for volumetric oxidation) involves heating oxide fuel with oxygen, sometimes with alternating oxidation and reduction, or alternating oxidation by ozone to uranium trioxide with decomposition by heating back to triuranium octoxide. A major purpose is to capture tritium
as tritiated water vapor before further processing where it would be
difficult to retain the tritium. Other volatile elements leave the fuel
and must be recovered, especially iodine, technetium, and carbon-14.
Voloxidation also breaks up the fuel or increases its surface area to
enhance penetration of reagents in following reprocessing steps.
Volatilization in isolation
Simply
heating spent oxide fuel in an inert atmosphere or vacuum at a
temperature between 700 °C and 1000 °C as a first reprocessing step can
remove several volatile elements, including caesium whose isotope caesium-137
emits about half of the heat produced by the spent fuel over the
following 100 years of cooling (however, most of the other half is from strontium-90 which remains).
Fluoride volatility
Blue
elements have volatile fluorides or are already volatile; green
elements do not but have volatile chlorides; red elements have neither,
but the elements themselves or their oxides are volatile at very high
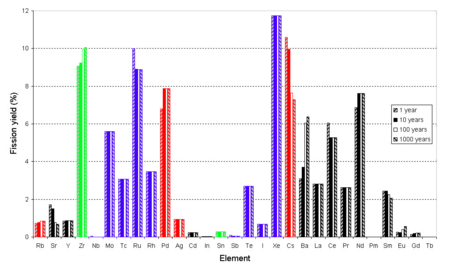
temperatures. Yields at 100,1,2,3 years after fission, not considering later neutron capture, fraction of 100% not 200%. Beta decay Kr-85→Rb, Sr-90→Zr, Ru-106→Pd, Sb-125→Te, Cs-137→Ba, Ce-144→Nd, Sm-151→Eu, Eu-155→Gd visible.
In the fluoride volatility process, fluorine is reacted with the fuel. Fluorine is so much more reactive than even oxygen
that small particles of ground oxide fuel will burst into flame when
dropped into a chamber full of fluorine. This is known as flame
fluorination; the heat produced helps the reaction proceed. Most of the uranium, which makes up the bulk of the fuel, is converted to uranium hexafluoride, the form of uranium used in uranium enrichment, which has a very low boiling point. Technetium, the main long-lived fission product,
is also efficiently converted to its volatile hexafluoride. A few other
elements also form similarly volatile hexafluorides, pentafluorides, or
heptafluorides. The volatile fluorides can be separated from excess
fluorine by condensation, then separated from each other by fractional distillation or selective reduction. Uranium hexafluoride and technetium hexafluoride have very similar boiling points and vapor pressures, which makes complete separation more difficult.
Many of the fission products volatilized are the same ones volatilized in non-fluorinated, higher-temperature volatilization, such as iodine, tellurium and molybdenum; notable differences are that technetium is volatilized, but caesium is not.
Some transuranium elements such as plutonium, neptunium and americium can form volatile fluorides, but these compounds are not stable when the fluorine partial pressure is decreased.
Most of the plutonium and some of the uranium will initially remain in
ash which drops to the bottom of the flame fluorinator. The
plutonium-uranium ratio in the ash may even approximate the composition
needed for fast neutron reactor fuel. Further fluorination of the ash can remove all the uranium, neptunium, and plutonium as volatile fluorides; however, some other minor actinides may not form volatile fluorides and instead remain with the alkaline fission products. Some noble metals may not form fluorides at all, but remain in metallic form; however ruthenium hexafluoride is relatively stable and volatile.
Distillation of the residue at higher temperatures can separate lower-boiling transition metal fluorides and alkali metal (Cs, Rb) fluorides from higher-boiling lanthanide and alkaline earth metal (Sr, Ba) and yttrium
fluorides. The temperatures involved are much higher, but can be
lowered somewhat by distilling in a vacuum. If a carrier salt like lithium fluoride or sodium fluoride is being used as a solvent, high-temperature distillation is a way to separate the carrier salt for reuse.
Molten salt reactor designs carry out fluoride volatility reprocessing continuously or at frequent intervals. The goal is to return actinides to the molten fuel mixture for eventual fission, while removing fission products that are neutron poisons, or that can be more securely stored outside the reactor core while awaiting eventual transfer to permanent storage.
Chloride volatility and solubility
Many of the elements that form volatile high-valence
fluorides will also form volatile high-valence chlorides. Chlorination
and distillation is another possible method for separation. The sequence
of separation may differ usefully from the sequence for fluorides; for
example, zirconium tetrachloride and tin tetrachloride
have relatively low boiling points of 331 °C and 114.1 °C. Chlorination
has even been proposed as a method for removing zirconium fuel
cladding, instead of mechanical decladding.
Chlorides are likely to be easier than fluorides to later convert back to other compounds, such as oxides.
Chlorides remaining after volatilization may also be separated by solubility in water. Chlorides of alkaline elements like americium, curium, lanthanides, strontium, caesium are more soluble than those of uranium, neptunium, plutonium, and zirconium.
Radioanalytical separations
In order to determine the distribution of radioactive metals for analytical purposes, Solvent Impregnated Resins (SIRs)
can be used. SIRs are porous particles, which contain an extractant
inside their pores. This approach avoids the liquid-liquid separation
step required in conventional liquid-liquid extraction.
For the preparation of SIRs for radioanalytical separations, organic
Amberlite XAD-4 or XAD-7 can be used. Possible extractants are e.g.
trihexyltetradecylphosphonium chloride(CYPHOS IL-101) or
N,N0-dialkyl-N,N0-diphenylpyridine-2,6-dicarboxyamides
(R-PDA; R = butyl, octy I, decyl, dodecyl).
Economics
The
relative economics of reprocessing-waste disposal and interim
storage-direct disposal was the focus of much debate over the first
decade of the 2000s. Studies
have modeled the total fuel cycle costs of a reprocessing-recycling system based on one-time recycling of plutonium in existing thermal reactors (as opposed to the proposed breeder reactor
cycle) and compare this to the total costs of an open fuel cycle with
direct disposal. The range of results produced by these studies is very
wide, but all are agreed that under current (2005) economic conditions
the reprocessing-recycle option is the more costly.
If reprocessing is undertaken only to reduce the radioactivity
level of spent fuel it should be taken into account that spent nuclear
fuel becomes less radioactive over time. After 40 years its
radioactivity drops by 99.9%, though it still takes over a thousand years for the level of radioactivity to approach that of natural uranium. However the level of transuranic elements,
including plutonium-239, remains high for over 100,000 years, so if not reused as nuclear fuel, then those elements need secure disposal because of nuclear proliferation reasons as well as radiation hazard.
On 25 October 2011 a commission of the Japanese Atomic Energy
Commission revealed during a meeting calculations about the costs of
recycling nuclear fuel for power generation. These costs could be twice
the costs of direct geological disposal of spent fuel: the cost of
extracting plutonium and handling spent fuel was estimated at 1.98 to
2.14 yen per kilowatt-hour of electricity generated. Discarding the
spent fuel as waste would cost only 1 to 1.35 yen per kilowatt-hour.
In July 2004 Japanese newspapers reported that the Japanese
Government had estimated the costs of disposing radioactive waste,
contradicting claims four months earlier that no such estimates had been
made. The cost of non-reprocessing options was estimated to be between a
quarter and a third ($5.5–7.9 billion) of the cost of reprocessing
($24.7 billion). At the end of the year 2011 it became clear that Masaya
Yasui, who had been director of the Nuclear Power Policy Planning
Division in 2004, had instructed his subordinate in April 2004 to
conceal the data. The fact that the data were deliberately concealed
obliged the ministry to re-investigate the case and to reconsider
whether to punish the officials involved.