Asymmetrical fire barrier, required by ASTM E119 to be fire tested on both sides. The lowest result achieved equates to the overall fire-resistance rating of the system in order to ensure that the barrier works equally well from both sides.
Asymmetry is the absence of, or a violation of, symmetry
(the property of an object being invariant to a transformation, such as
reflection). Symmetry is an important property of both physical and
abstract systems and it may be displayed in precise terms or in more
aesthetic terms. The absence of or violation of symmetry that are either
expected or desired can have important consequences for a system.
In organisms
Due to how cells divide in organisms, asymmetry in organisms is fairly usual in at least one dimension, with biological symmetry also being common in at least one dimension.
Louis Pasteur proposed that biological molecules are asymmetric
because the cosmic [i.e. physical] forces that preside over their
formation are themselves asymmetric. While at his time, and even now,
the symmetry of physical processes are highlighted, it is known that
there are fundamental physical asymmetries, starting with time.
Asymmetry in biology
Asymmetry is an important and widespread trait, having evolved
numerous times in many organisms and at many levels of organisation
(ranging from individual cells, through organs, to entire body-shapes).
Benefits of asymmetry sometimes have to do with improved spatial
arrangements, such as the left human lung being smaller, and having one fewer lobes than the right lung to make room for the asymmetrical heart.
In other examples, division of function between the right and left half
may have been beneficial and has driven the asymmetry to become
stronger. Such an explanation is usually given for mammal hand or paw
preference (Handedness),
an asymmetry in skill development in mammals. Training the neural
pathways in a skill with one hand (or paw) may take less effort than
doing the same with both hands.
Nature also provides several examples of handedness in traits
that are usually symmetric. The following are examples of animals with
obvious left-right asymmetries:
Fiddler crab, Uca pugnax
- Most snails, because of torsion during development, show remarkable asymmetry in the shell and in the internal organs.
- Fiddler crabs have one big claw and one small claw.
- The narwhal's tusk is a left incisor which can grow up to 10 feet in length and forms a left-handed helix.
- Flatfish have evolved to swim with one side upward, and as a result have both eyes on one side of their heads.
- Several species of owls exhibit asymmetries in the size and positioning of their ears, which is thought to help locate prey.
- Many animals (ranging from insects to mammals) have asymmetric male genitalia. The evolutionary cause behind this is, in most cases, still a mystery.
As an indicator of unfitness
- Certain disturbances during the development of the organism, resulting in birth defects.
- Injuries after cell division that cannot be biologically repaired, such as a lost limb from an accident.
Since birth defects and injuries are likely to indicate poor health
of the organism, defects resulting in asymmetry often put an animal at a
disadvantage when it comes to finding a mate. In particular, a degree
of facial symmetry is associated with physical attractiveness, but complete symmetry is both impossible and probably unattractive.
In structures
Pre-modern
architectural styles tended to place an emphasis on symmetry, except
where extreme site conditions or historical developments lead away from
this classical ideal. To the contrary, modernist and postmodern architects became much more free to use asymmetry as a design element.
While most bridges employ a symmetrical form due to intrinsic
simplicities of design, analysis and fabrication and economical use of
materials, a number of modern bridges have deliberately departed from
this, either in response to site-specific considerations or to create a
dramatic design statement.
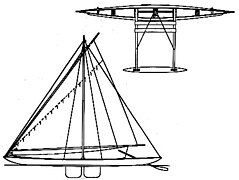
Some asymmetrical structures
- A proa, a form of outrigger canoe
In fire protection
In fire-resistance rated wall assemblies, used in passive fire protection, including, but not limited to, high-voltage transformer fire barriers, asymmetry is a crucial aspect of design. When designing a facility, it is not always certain, that in the event of fire, which side
a fire may come from. Therefore, many building codes and fire test
standards outline, that a symmetrical assembly, need only be tested
from one side, because both sides are the same. However, as soon as an
assembly is asymmetrical, both sides must be tested and the test report
is required to state the results for each side. In practical use, the
lowest result achieved is the one that turns up in certification listings.
Neither the test sponsor, nor the laboratory can go by an opinion or
deduction as to which side was in more peril as a result of contemplated
testing and then test only one side. Both must be tested in order to be
compliant with test standards and building codes.
In mathematics
There are no a and b such that a < b and b < a. This form of asymmetry is an asymmetrical relation.
In chemistry
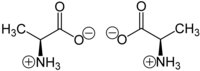
Certain molecules are chiral;
that is, they cannot be superposed upon their mirror image. Chemically
identical molecules with different chirality are called enantiomers; this difference in orientation can lead to different properties in the way they react with biological systems.
In physics
Asymmetry arises in physics in a number of different realms.
Thermodynamics
The original non-statistical formulation of thermodynamics was asymmetrical in time: it claimed that the entropy in a closed system can only increase with time. This was derived from the Second Law (any of the two, Clausius' or Lord Kelvin's statement can be used since they are equivalent) and using the Clausius' Theorem (see Kerson Huang ISBN 978-0471815181).
The later theory of statistical mechanics, however, is symmetric in
time. Although it states that a system significantly below maximum
entropy is very likely to evolve towards higher entropy, it also states that such a system is very likely to have evolved from higher entropy.
Particle physics
Symmetry is one of the most powerful tools in particle physics,
because it has become evident that practically all laws of nature
originate in symmetries. Violations of symmetry therefore present
theoretical and experimental puzzles that lead to a deeper understanding
of nature. Asymmetries in experimental measurements also provide
powerful handles that are often relatively free from background or
systematic uncertainties.
Parity violation
Until the 1950s, it was believed that fundamental physics was
left-right symmetric; i.e., that interactions were invariant under parity. Although parity is conserved in electromagnetism, strong interactions and gravity, it turns out to be violated in weak interactions. The Standard Model incorporates parity violation by expressing the weak interaction as a chiral
gauge interaction. Only the left-handed components of particles and
right-handed components of antiparticles participate in weak
interactions in the Standard Model. A consequence of parity violation in
particle physics is that neutrinos have only been observed as left-handed particles (and antineutrinos as right-handed particles).
In 1956-1957 Chien-Shiung Wu,
E. Ambler, R. W. Hayward, D. D. Hoppes, and R. P. Hudson found a clear
violation of parity conservation in the beta decay of cobalt-60. Simultaneously, R. L. Garwin, Leon Lederman, and R. Weinrich modified an existing cyclotron experiment and immediately verified parity violation.
CP violation
After the discovery of the violation of parity in 1956-57, it was
believed that the combined symmetry of parity (P) and simultaneous charge conjugation (C), called CP, was preserved. For example, CP transforms a left-handed neutrino into a right-handed antineutrino. In 1964, however, James Cronin and Val Fitch provided clear evidence that CP symmetry was also violated in an experiment with neutral kaons.
CP violation is one of the necessary conditions for the generation of a baryon asymmetry in the universe.
Combining the CP symmetry with simultaneous time reversal (T) produces a combined symmetry called CPT symmetry. CPT symmetry must be preserved in any Lorentz invariant local quantum field theory with a Hermitian Hamiltonian. As of 2006, no violations of CPT symmetry have been observed.
Baryon asymmetry of the universe
The baryons (i.e., the protons and neutrons and the atoms that they comprise) observed so far in the universe are overwhelmingly matter as opposed to anti-matter. This asymmetry is called the baryon asymmetry of the universe.
Isospin violation
Isospin is the symmetry transformation of the weak interactions. The concept was first introduced by Werner Heisenberg in nuclear physics based on the observations that the masses of the neutron and the proton are almost identical and that the strength of the strong interaction
between any pair of nucleons is the same, independent of whether they
are protons or neutrons. This symmetry arises at a more fundamental
level as a symmetry between up-type and down-type quarks. Isospin symmetry in the strong interactions can be considered as a subset of a larger flavor symmetry group, in which the strong interactions are invariant under interchange of different types of quarks. Including the strange quark in this scheme gives rise to the Eightfold Way scheme for classifying mesons and baryons.
Isospin is violated by the fact that the masses of the up and
down quarks are different, as well as by their different electric
charges. Because this violation is only a small effect in most processes
that involve the strong interactions, isospin symmetry remains a useful
calculational tool, and its violation introduces corrections to the
isospin-symmetric results.
In collider experiments
Because the weak interactions violate parity, collider
processes that can involve the weak interactions typically exhibit
asymmetries in the distributions of the final-state particles. These
asymmetries are typically sensitive to the difference in the
interaction between particles and antiparticles, or between left-handed
and right-handed particles. They can thus be used as a sensitive
measurement of differences in interaction strength and/or to distinguish
a small asymmetric signal from a large but symmetric background.
- A forward-backward asymmetry is defined as AFB=(NF-NB)/(NF+NB), where NF is the number of events in which some particular final-state particle is moving "forward" with respect to some chosen direction (e.g., a final-state electron moving in the same direction as the initial-state electron beam in electron-positron collisions), while NB is the number of events with the final-state particle moving "backward". Forward-backward asymmetries were used by the LEP experiments to measure the difference in the interaction strength of the Z boson between left-handed and right-handed fermions, which provides a precision measurement of the weak mixing angle.
- A left-right asymmetry is defined as ALR=(NL-NR)/(NL+NR), where NL is the number of events in which some initial- or final-state particle is left-polarized, while NR is the corresponding number of right-polarized events. Left-right asymmetries in Z boson production and decay were measured at the Stanford Linear Collider using the event rates obtained with left-polarized versus right-polarized initial electron beams. Left-right asymmetries can also be defined as asymmetries in the polarization of final-state particles whose polarizations can be measured; e.g., tau leptons.
- A charge asymmetry or particle-antiparticle asymmetry is defined in a similar way. This type of asymmetry has been used to constrain the parton distribution functions of protons at the Tevatron from events in which a produced W boson decays to a charged lepton. The asymmetry between positively and negatively charged leptons as a function of the direction of the W boson relative to the proton beam provides information on the relative distributions of up and down quarks in the proton. Particle-antiparticle asymmetries are also used to extract measurements of CP violation from B meson and anti-B meson production at the BaBar and Belle experiments.
Lexical
Asymmetry is also relevant to grammar and linguistics, especially in the contexts of lexical analysis and transformational grammar.
Enumeration example:
In English, there are grammatical rules for specifying coordinate items in an enumeration or series. Similar rules exist for programming languages and mathematical notation. These rules vary, and some require lexical asymmetry to be considered grammatically correct.
For example, in standard written English:
We sell domesticated cats, dogs, and goldfish. ### in-line asymmetric and grammatical
We sell domesticated animals (cats, dogs, goldfish). ### in-line symmetric and grammatical
We sell domesticated animals (cats, dogs, goldfish,). ### in-line symmetric and ungrammatical
We sell domesticated animals: ### outline symmetric and grammatical
- cats
- dogs
- goldfish